Humane pluripotentne NT2/D1 ćelije imobilizovane u alginatnim mikrovlaknima: 3D sistem za testiranje uticaja bioaktivnih jedinjenja Naučni rad
Glavni sadržaj članka
Apstrakt
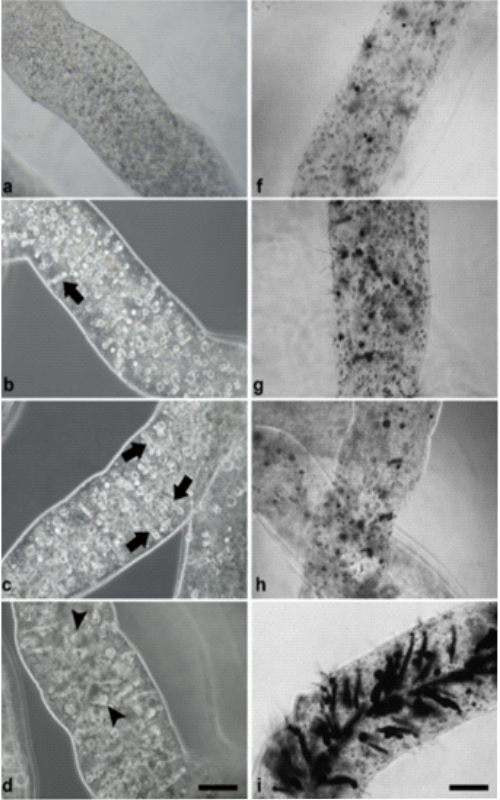
Učestala konzumacija energetskih pića (engl. energy drinks-ED) i njihova kombinacija sa alkoholom (engl. energy drinks mixed with alcohol-AmED) postala je rastući trend među mladim ljudima. Proklamovano povećanje psihičkih i fizičkih performansi usled konzumiranja ED, koji u svom sastavu sadrže visoke doze stimulativnih sastojka - kofeina i taurina, može dovesti do neželjenih posledica, prvenstveno na funkcije centralnog nervnog sistema (CNS) i kardiovaskularnog sistema. Uprkos ključnoj ulozi u razviću i adultnoj homeostazi, istraživanja o potencijalnom efektu konzumacije ED i AmED na matične i progenitorske ćelije su malobrojne. U radu je predstavljena optimizacija 3D model sistema na bazi alginatnih mikrovlakana za testiranje uticaja bioaktivnih jedinjenja na humane NT2/D1 embrionalne karcinomske ćelije, široko korišćeni model sistem kao pandan humanim matičnim ćelijama. Ispitan je uticaj akutne konzumacije ED i AmED na vijabilnost ovih ćelija i uz pomoć matematičkog modelovanja evaluirana je efikasnost prenosa mase ispitivanih komponenti do imobilisanih ćelija. Dobijeni rezultati pokazuju da ovaj model sistem omogućava optimalan rast i proliferaciju NT2/D1 pluripotentnih ćelija i uniformnu distribuciju ispitivanih komponenti kroz alginatna mikrovlakna. Simulirana akutna konzumacija ED i AmED nije uticala na vijabilnost NT2/D1 ćelija u 3D sistemu, za razliku od 2D modela gde je kofein doveo do malog ali statistički značajnog pada vijabilnosti.
Detalji članka
Broj časopisa
Rubrika

Ovaj rad je pod Creative Commons Autorstvo 4.0 Internacionalna licenca.
Kada je rukopis prihvaćen za objavlјivanje, autori prenose autorska prava na izdavača. U slučaju da rukopis ne bude prihvaćen za štampu u časopisu, autori zadržavaju sva prava.
Na izdavača se prenose sledeća prava na rukopis, uklјučujući i dodatne materijale, i sve delove, izvode ili elemente rukopisa:
- pravo da reprodukuje i distribuira rukopis u štampanom obliku, uklјučujući i štampanje na zahtev;
- pravo na štampanje probnih primeraka, reprint i specijalnih izdanja rukopisa;
- pravo da rukopis prevede na druge jezike;
- pravo da rukopis reprodukuje koristeći fotomehanička ili slična sredstva, uklјučujući, ali ne ograničavajući se na fotokopiranje, i pravo da distribuira ove kopije;
- pravo da rukopis reprodukuje i distribuira elektronski ili optički koristeći sve nosioce podataka ili medija za pohranjivanje, a naročito u mašinski čitlјivoj/digitalizovanoj formi na nosačima podataka kao što su hard disk, CD-ROM, DVD, Blu-ray Disc (BD), mini disk, trake sa podacima, i pravo da reprodukuje i distribuira rukopis sa tih prenosnika podataka;
- pravo da sačuva rukopis u bazama podataka, uklјučujući i onlajn baze podataka, kao i pravo prenosa rukopisa u svim tehničkim sistemima i režimima;
- pravo da rukopis učini dostupnim javnosti ili zatvorenim grupama korisnika na osnovu pojedinačnih zahteva za upotrebu na monitoru ili drugim čitačima (uklјučujući i čitače elektonskih knjiga), i u štampanoj formi za korisnike, bilo putem interneta, onlajn servisa, ili putem internih ili eksternih mreža.
Kako citirati
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-66/2024-03/200042;451-03-65/2024-03/200135;451-03-66/2024-03/200287
Reference
[1] Alsunni AA. Energy Drink Consumption: Beneficial and Adverse Health Effects. Int J Health Sci (Qassim). 2015; 9(4): 468-474. https://ijhs.qu.edu.sa/index.php/journal/article/view/1244
[2] Nadeem IM, Shanmugaraj A, Sakha S, Horner NS, Ayeni OR, Khan M. Energy Drinks and Their Adverse Health Effects: A Systematic Review and Meta-analysis. Sports Health. 2021; 13(3): 265-277. https://doi.org/10.1177/1941738120949181PMC8083152
[3] De Sanctis V, Soliman N, Soliman AT, Elsedfy H, Di Maio S, El Kholy M, Fiscina B. Caffeinated energy drink consumption among adolescents and potential health consequences associated with their use: a significant public health hazard. Acta Biomed. 2017; 88(2): 222-231. https://doi.org/10.23750/abm.v88i2.6664PMC6166148
[4] Ding M, Markon AO, Jones-Dominic OE, Purdue-Smithe AC, Rich-Edwards JW, Wolpert BJ, Chavarro JE. Intake of Energy Drinks Before and During Pregnancy and Adverse Pregnancy Outcomes. JAMA Netw Open. 2023; 6(11): e2344023. https://doi.org/10.1001/jamanetworkopen.2023.44023PMC10660164
[5] Oteri A, Salvo F, Caputi AP, Calapai G. Intake of energy drinks in association with alcoholic beverages in a cohort of students of the School of Medicine of the University of Messina. Alcohol Clin Exp Res. 2007; 31(10): 1677-1680. https://doi.org/10.1111/j.1530-0277.2007.00464.x
[6] Luo YS, Chen Z, Blanchette AD, Zhou YH, Wright FA, Baker ES, Chiu WA, Rusyn I. Relationships between constituents of energy drinks and beating parameters in human induced pluripotent stem cell (iPSC)-Derived cardiomyocytes. Food Chem Toxicol. 2021; 149: 111979. https://doi.org/10.1016/j.fct.2021.111979PMC8286543
[7] Serdar M, Mordelt A, Müser K, Kempe K, Felderhoff-Müser U, Herz J, Bendix I. Detrimental Impact of Energy Drink Compounds on Developing Oligodendrocytes and Neurons. Cells. 2019; 8(11): 1381. https://doi.org/10.3390/cells8111381PMC6912672
[8] Zeidán-Chuliá F, Gelain DP, Kolling EA, Rybarczyk-Filho JL, Ambrosi P, Terra SR, Pires AS, da Rocha JB, Behr GA, Moreira JC. Major components of energy drinks (caffeine, taurine, and guarana) exert cytotoxic effects on human neuronal SH-SY5Y cells by decreasing reactive oxygen species production. Oxid Med Cell Longev. 2013; 2013: 791795. https://doi.org/10.1155/2013/791795PMC3674721
[9] Andrews PW. From teratocarcinomas to embryonic stem cells. Philos Trans R Soc Lond B Biol Sci. 2002; 357(1420): 405-417. https://doi.org/10.1098/rstb.2002.1058PMC1692959
[10] Andrews PW, Damjanov I, Simon D, Banting GS, Carlin C, Dracopoli NC, Føgh J. Pluripotent embryonal carcinoma clones derived from the human teratocarcinoma cell line Tera-2. Differentiation in vivo and in vitro. Lab Invest. 1984; 50(2): 147-162. https://pubmed.ncbi.nlm.nih.gov/6694356/
[11] Sperger JM, Chen X, Draper JS, Antosiewicz JE, Chon CH, Jones SB, Brooks JD, Andrews PW, Brown PO, Thomson JA. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc Natl Acad Sci U S A. 2003; 100(23): 13350-13355. https://doi.org/10.1073/pnas.2235735100PMC263817
[12] Foglizzo V, Cocco E, Marchiò S. Advanced Cellular Models for Preclinical Drug Testing: From 2D Cultures to Organ-on-a-Chip Technology. Cancers (Basel). 2022; 14(15): 3692. https://doi.org/10.3390/cancers14153692PMC9367322
[13] Kapałczyńska M, Kolenda T, Przybyła W, Zajączkowska M, Teresiak A, Filas V, Ibbs M, Bliźniak R, Łuczewski Ł, Lamperska K. 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch Med Sci. 2018; 14(4): 910-919. https://doi.org/10.5114/aoms.2016.63743PMC6040128
[14] Habanjar O, Diab-Assaf M, Caldefie-Chezet F, Delort L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int J Mol Sci. 2021; 22(22): 12200. https://doi.org/10.3390/ijms222212200PMC8618305
[15] Zhao X, Bhattacharyya A. Human Models Are Needed for Studying Human Neurodevelopmental Disorders. Am J Hum Genet. 2018; 103(6): 829-857. https://doi.org/10.1016/j.ajhg.2018.10.009PMC6288051
[16] Ginis I, Luo Y, Miura T, Thies S, Brandenberger R, Gerecht-Nir S, Amit M, Hoke A, Carpenter MK, Itskovitz-Eldor J, Rao MS. Differences between human and mouse embryonic stem cells. Dev Biol. 2004; 269(2): 360-380. https://doi.org/10.1016/j.ydbio.2003.12.034
[17] Greber B, Wu G, Bernemann C, Joo JY, Han DW, Ko K, Tapia N, Sabour D, Sterneckert J, Tesar P, Schöler HR. Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells. Cell Stem Cell. 2010; 6(3): 215-226. https://doi.org/10.1016/j.stem.2010.01.003
[18] Matsuda M, Hayashi H, Garcia-Ojalvo J, Yoshioka-Kobayashi K, Kageyama R, Yamanaka Y, Ikeya M, Toguchida J, Alev C, Ebisuya M. Species-specific segmentation clock periods are due to differential biochemical reaction speeds. Science. 2020; 369(6510): 1450-1455. https://doi.org/doi:10.1126/science.aba7668
[19] European Parliament and of the Council. Consolidated text: Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off J Eur Union. 2010; L 276: 233–279. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02010L0063-20190626
[20] Hill EJ, Woehrling EK, Prince M, Coleman MD. Differentiating human NT2/D1 neurospheres as a versatile in vitro 3D model system for developmental neurotoxicity testing. Toxicology. 2008; 249 (2-3): 243-250. https://doi.org/10.1016/j.tox.2008.05.014
[21] Cacciotti I, Ceci C, Bianco A, Pistritto G. Neuro-differentiated Ntera2 cancer stem cells encapsulated in alginate beads: First evidence of biological functionality. Mater Sci Eng C Mater Biol Appl. 2017; 81: 32-38. https://doi.org/10.1016/j.msec.2017.07.033
[22] Radonjić M, Petrović J, Milivojević M, Stevanović M, Stojkovska J, Obradović B. Chemical engineering methods in analyses of 3D cancer cell cultures: Hydrodinamic and mass transport considerations. Chem Ind Chem Eng Q. 2022; 28(3): 211-223. https://doi.org/10.2298/CICEQ210607033R
[23] Sun J, Tan H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials (Basel). 2013; 6(4): 1285-1309. https://doi.org/10.3390/ma6041285PMC5452316
[24] Mead EA, Wang Y, Patel S, Thekkumthala AP, Kepich R, Benn-Hirsch E, Lee V, Basaly A, Bergeson S, Siegelmann HT, Pietrzykowski AZ. miR-9 utilizes precursor pathways in adaptation to alcohol in mouse striatal neurons. Adv Drug Alcohol Res. 2023; 3: 11323. https://doi.org/10.3389/adar.2023.11323PMC10730111
[25] Doyle W, Shide E, Thapa S, Chandrasekaran V. The effects of energy beverages on cultured cells. Food Chem Toxicol. 2012; 50(10): 3759-3768. https://doi.org/10.1016/j.fct.2012.07.008
[26] Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65(1-2): 55-63. https://doi.org/10.1016/0022-1759(83)90303-4
[27] Stojkovska JJ, Zvicer J, Milivojevic M, Petrovic I, Stevanovic M, Obradovic B. Validation of a novel perfusion bioreactor system in cancer research. Hem Ind. 2020; 74(3): 187-196. https://doi.org/10.2298/HEMIND200329015S
[28] Saltzman WM, Kyriakides TR. Cell interactions with polymers. In: Lanza R, Langer R, Vacanti JP, Atala A, eds. Principles of Tissue Engineering 5th ed. Cambridge, MA: Academic Press; 2020: 275-293. https://doi.org/10.1016/B978-0-12-818422-6.00017-4
[29] Nebel S, Lux M, Kuth S, Bider F, Dietrich W, Egger D, Boccaccini AR, Kasper C. Alginate Core-Shell Capsules for 3D Cultivation of Adipose-Derived Mesenchymal Stem Cells. Bioengineering (Basel). 2022; 9(2): 66. https://doi.org/10.3390/bioengineering9020066PMC8869374
[30] Rodriguez S, Lau H, Corrales N, Heng J, Lee S, Stiner R, Alexander M, Lakey JRT. Characterization of chelator-mediated recovery of pancreatic islets from barium-stabilized alginate microcapsules. Xenotransplantation. 2020; 27(1): e12554. https://doi.org/10.1111/xen.12554
[31] Vo P, Nguyen S, Minh Do N, Truong K, Pham P. Sodium citrate inhibits proliferation and induces apoptosis of hepatocellular carcinoma cells. Biomed Res Ther. 2020; 7: 3659-3666. https://doi.org/10.15419/bmrat.v7i3.592
[32] Wu Y, Jia C, Liu W, Zhan W, Chen Y, Lu J, Bao Y, Wang S, Yu C, Zheng L, Sun L, Song Z. Sodium citrate targeting Ca(2+)/CAMKK2 pathway exhibits anti-tumor activity through inducing apoptosis and ferroptosis in ovarian cancer. J Adv Res. 2024; 65: 89-104. https://doi.org/10.1016/j.jare.2024.04.033PMC11518946
[33] Burger H, Nooter K, Boersma AW, Kortland CJ, Stoter G. Expression of p53, Bcl-2 and Bax in cisplatin-induced apoptosis in testicular germ cell tumour cell lines. Br J Cancer. 1998; 77(10): 1562-1567. https://doi.org/10.1038/bjc.1998.257PMC2150079
[34] Kitano O, Nakazawa K. Neuronal Differentiation of NT2 Cells in Monolayer and Spheroid Cultures. MATEC Web Conf. 2021; 333: 07008. https://doi.org/10.1051/matecconf/202133307008
[35] Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol. 2010; 148(1): 3-15. https://doi.org/10.1016/j.jbiotec.2010.01.012
[36] Pinto B, Henriques AC, Silva PMA, Bousbaa H. Three-Dimensional Spheroids as In Vitro Preclinical Models for Cancer Research. Pharmaceutics. 2020; 12(12): 1186. https://doi.org/10.3390/pharmaceutics12121186PMC7762220
[37] Han YL, Wang S, Zhang X, Li Y, Huang G, Qi H, Pingguan-Murphy B, Li Y, Lu TJ, Xu F. Engineering physical microenvironment for stem cell based regenerative medicine. Drug Discov Today. 2014; 19(6): 763-773. https://doi.org/10.1016/j.drudis.2014.01.015
[38] Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev. 2008; 14(2): 149-165. https://doi.org/10.1089/ten.teb.2007.0332PMC2962861
[39] Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009; 103(4): 655-663. https://doi.org/10.1002/bit.22361PMC2997742
[40] Vergani L, Grattarola M, Nicolini C. Modifications of chromatin structure and gene expression following induced alterations of cellular shape. Int J Biochem Cell Biol. 2004; 36(8): 1447-1461. https://doi.org/10.1016/j.biocel.2003.11.015
[41] Demircan Yalcin Y, Luttge R. Electrical monitoring approaches in 3-dimensional cell culture systems: Toward label-free, high spatiotemporal resolution, and high-content data collection in vitro. Organs-on-a-Chip. 2021; 3: 100006. https://doi.org/https://doi.org/10.1016/j.ooc.2021.100006
[42] Hagiwara M, Nobata R, Kawahara T. High repeatability from 3D experimental platform for quantitative analysis of cellular branch pattern formations. Integr Biol (Camb). 2018; 10(5): 306-312. https://doi.org/10.1039/c8ib00032h
[43] Jensen C, Teng Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front Mol Biosci. 2020; 7: 33. https://doi.org/10.3389/fmolb.2020.00033PMC7067892
[44] Eftekharzadeh B, Khodagholi F, Abdi A, Maghsoudi N. Alginate protects NT2 neurons against H2O2-induced neurotoxicity. Carbohydr Polym. 2010; 79(4): 1063-1072. https://doi.org/https://doi.org/10.1016/j.carbpol.2009.10.040
[45] Feng WJ, Mou J, Liao PP, Zhou J, Zhang NN, Hu T, Wang S, Zhang SY, Mao YJ. Alginate oligosaccharides exert protective effects on hydrogen peroxide-induced senescence in H9C2 cardiomyocytes by regulating the redox state of cells. Food Sci Biotechnol. 2024; 33(12): 2835-2844. https://doi.org/10.1007/s10068-024-01534-yPMC11339193
[46] Saleh EM, Hamdy GM, Hassan RE. Neuroprotective effect of sodium alginate against chromium-induced brain damage in rats. PLoS One. 2022; 17(4): e0266898. https://doi.org/10.1371/journal.pone.0266898PMC9009676
[47] Krahe TE, Filgueiras CC, da Silva Quaresma R, Schibuola HG, Abreu-Villaça Y, Manhães AC, Ribeiro-Carvalho A. Energy drink enhances the behavioral effects of alcohol in adolescent mice. Neurosci Lett. 2017; 651: 102-108. https://doi.org/https://doi.org/10.1016/j.neulet.2017.04.050
[48] Richards G, Smith AP. A Review of Energy Drinks and Mental Health, with a Focus on Stress, Anxiety, and Depression. J Caffeine Res. 2016; 6(2): 49-63. https://doi.org/10.1089/jcr.2015.0033PMC4892220
[49] Graneri L, Lam V, D'Alonzo Z, Nesbit M, Mamo JCL, Takechi R. The Consumption of Energy Drinks Induces Blood-Brain Barrier Dysfunction in Wild-Type Mice. Front Nutr. 2021; 8: 668514. https://doi.org/10.3389/fnut.2021.668514PMC8126614
[50] Petribu BN, Abrahao KP, Souza-Formigoni MLO. Ethanol combined with energy drinks: Two decades of research in rodents. Front Behav Neurosci. 2022; 16: 1100608. https://doi.org/10.3389/fnbeh.2022.1100608PMC10017554
[51] MATLAB Release 2018a [computer program]. Natick, MA: The MathWorks, Inc.; 2018. https://www.mathworks.com/
[52] Chern J-M, Lee W-F, Hsieh M-Y. Absorption Isotherm of Caffeine and Release Kinetics from Swollen NIPAAm Hydrogels: Experiments and Modeling. Ind Eng Chem Res. 2004; 43(19): 6150-6156. https://doi.org/10.1021/ie049616d
[53] Rodak K, Kokot I, Kratz EM. Caffeine as a Factor Influencing the Functioning of the Human Body-Friend or Foe? Nutrients. 2021; 13(9): 3088. https://doi.org/10.3390/nu13093088PMC8467199
[54] Estapé D, Gòdia F, Solà C. Determination of glucose and ethanol effective diffusion coefficients in Ca-alginate gel. Enzyme Microb Technol. 1992; 14(5): 396-401. https://doi.org/10.1016/0141-0229(92)90009-d
[55] Jain M, Nilsson R, Sharma S, Madhusudhan N, Kitami T, Souza AL, Kafri R, Kirschner MW, Clish CB, Mootha VK. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012; 336(6084): 1040-1044. https://doi.org/10.1126/science.1218595PMC3526189
[56] Zhao F, Pathi P, Grayson W, Xing Q, Locke BR, Ma T. Effects of oxygen transport on 3-d human mesenchymal stem cell metabolic activity in perfusion and static cultures: experiments and mathematical model. Biotechnol Prog. 2005; 21(4): 1269-1280. https://doi.org/10.1021/bp0500664
[57] Urzì O, Gasparro R, Costanzo E, De Luca A, Giavaresi G, Fontana S, Alessandro R. Three-Dimensional Cell Cultures: The Bridge between In Vitro and In Vivo Models. Int J Mol Sci. 2023; 24(15): 12046. https://doi.org/10.3390/ijms241512046PMC10419178
[58] Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol. 1984; 103(2): 285-293. https://doi.org/10.1016/0012-1606(84)90316-6