Uticaj predtretmana, parametara liofilizacije, korišćenja različitih krioprotektanata na preživljavanje imobilisanih probiotika tokom postupka liofilizacije Naučni rad
Glavni sadržaj članka
Apstrakt
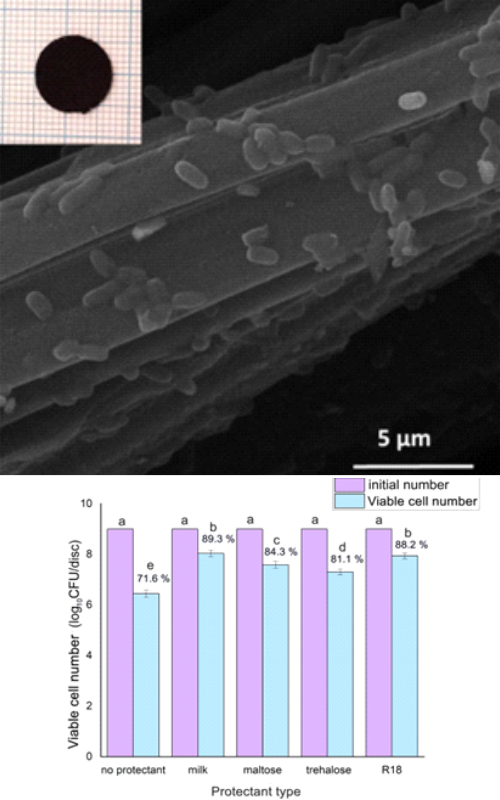
Liofilizacija je odlična metoda koja omogućavo produženje roka trajanja prehrambenih proizvoda kao i očuvanje probiotskih kultura. Žive ćelije preferiraju blage uslove i svako odstupanje (vakum, visoka ili niska temperatura) dovodi do oštećenja ćelija. Ovaj rad ispituje kako različiti parametri kao što su predtretmani, trajanje liofilizacije, tip smrzavanja, primena krioprotektanata utiče na preživljavanje probiotika imobilisanih na tkaninu od aktivnog uglja tokom procesa liofilizacije. Aktivacija L. plantarum u MRS bujonu pre imobilizacije značajno povećava broj živih ćelija, ali i njihovo preživljavanje tokom liofilizacije. Zamrzavanje imobilisanih ćelija tečnim azotom nije dalo željeno povećanje procenta preživelih probiotskih ćelija nakon liofilizacije u poređenju sa dubokim smrzavanjem na -80°C, dok je inkubacija ćelija 2h u frizideru pre dubokog smrzavanja doprinela povećanju broja ćelija koje prežive liofilizaciju. Dužina trajanja (5 i 48h) samog procesa sušenja imala je blagi uticaj na stepen preživljavanja probiotika. Upotreba mleka kao krioprotektanta značajno je povećala stepen preživljavanja, dok su saharoza, maltoza i trehaloza pokazale dobru zaštitnu moć, ali znatno manju u poređenju sa mlekom. Najviši stepen preživljavanja L. plantarum obezbeđuje procedura koja podrazumeva inkubaciju ćelija u MRS bujonu, korišćenje mleka kao krioprotektanta, inkubaciju 2h u frizideru, duboko smrzavanje i liofilizaciju u trajanju od 5h.
Detalji članka
Broj časopisa
Rubrika

Ovaj rad je pod Creative Commons Autorstvo 4.0 Internacionalna licenca.
Kada je rukopis prihvaćen za objavlјivanje, autori prenose autorska prava na izdavača. U slučaju da rukopis ne bude prihvaćen za štampu u časopisu, autori zadržavaju sva prava.
Na izdavača se prenose sledeća prava na rukopis, uklјučujući i dodatne materijale, i sve delove, izvode ili elemente rukopisa:
- pravo da reprodukuje i distribuira rukopis u štampanom obliku, uklјučujući i štampanje na zahtev;
- pravo na štampanje probnih primeraka, reprint i specijalnih izdanja rukopisa;
- pravo da rukopis prevede na druge jezike;
- pravo da rukopis reprodukuje koristeći fotomehanička ili slična sredstva, uklјučujući, ali ne ograničavajući se na fotokopiranje, i pravo da distribuira ove kopije;
- pravo da rukopis reprodukuje i distribuira elektronski ili optički koristeći sve nosioce podataka ili medija za pohranjivanje, a naročito u mašinski čitlјivoj/digitalizovanoj formi na nosačima podataka kao što su hard disk, CD-ROM, DVD, Blu-ray Disc (BD), mini disk, trake sa podacima, i pravo da reprodukuje i distribuira rukopis sa tih prenosnika podataka;
- pravo da sačuva rukopis u bazama podataka, uklјučujući i onlajn baze podataka, kao i pravo prenosa rukopisa u svim tehničkim sistemima i režimima;
- pravo da rukopis učini dostupnim javnosti ili zatvorenim grupama korisnika na osnovu pojedinačnih zahteva za upotrebu na monitoru ili drugim čitačima (uklјučujući i čitače elektonskih knjiga), i u štampanoj formi za korisnike, bilo putem interneta, onlajn servisa, ili putem internih ili eksternih mreža.
Kako citirati
Funding data
-
Science Fund of the Republic of Serbia
Grant numbers Grant No 9802
Reference
[1] Malik KA. Survival and stability of microorganisms during freeze-drying. Cryobiology. 1988; 25(6): 517-518. https://doi.org/10.1016/0011-2240(88)90324-0
[2] Champagne CP, Gardner N., Brochu E., Beaulieu Y. The freeze-drying of lactic acid bacteria. Can Inst Food Technol J. 1991; 24: 118-128. https://doi.org/10.1016/S0315-5463(91)70034-5
[3] Hubálek Z. Protectants used in the cryopreservation of microorganisms. Cryobiology. 2003; 2003; 46: 205-229. https://doi.org/10.1016/S0011-2240(03)00046-4
[4] Alonso S. Novel Preservation Techniques for Microbial Cultures. in Ojha K, Tiwari B, eds. Novel Food Fermentation Technologies. Food Engineering Series. Springer; 2016: 8-33. https://doi.org/10.1007/978-3-319-42457-6_2
[5] Carvalho AS, Silva J, Ho P, Teixeira P, Malcata FX, Gibbs P. Relevant factors for the preparation of freeze-dried lactic acid bacteria. Int Dairy J. 2004; 14: 835-847. https://doi.org/10.1016/j.idairyj.2004.02.001
[6] Carvalho AS, Silva J, Ho P, Teixeira P, Malcata FX, Gibbs P. Survival of freeze-dried Lactobacillus plantarum and Lactobacillus rhamnosus during storage in the presence of protectants. Biotechnol Lett. 2002; 24: 1587-1591. https://link.springer.com/article/10.1023/A:1020301614728
[7] Morgan CA, Herman N, White PA, Vesey G. Preservation of micro-organisms by drying. J Microbiol Methods. 2006; 66: 183-193. https://doi.org/10.1016/j.mimet.2006.02.017
[8] Bouabidi ZB, El-Naas MH, Zhang Z. Immobilization of microbial cells for the biotreatment of wastewater. Environ Chem Lett. 2019; 17: 241-257. https://doi.org/10.1007/s10311-018-0795-7
[9] Wu P, Wang Z, Bhatnagar A, Jeyakumar P, Wang H, Wang Y, Li X, Microorganisms-carbonaceous materials immobilized complexes: Synthesis, adaptability and environmental applications. J Hazard Mater. 2021; 416: 125915. https://doi.org/10.1016/j.jhazmat.2021.125915
[10] Partovinia A, Rasekh B. Review of the immobilized microbial cell systems for bioremediation of petroleum hydrocarbons polluted environments. Crit Rev Environ Sci Technol. 2018; 48 (1): 1-38. https://doi.org/10.1080/10643389.2018.1439652
[11] Gong W, Fan Y, Xie B, Tang X, Guo T, Luo L, Liang H. Immobilizing Microcystis aeruginosa and powdered activated carbon for the anaerobic digestate effluent treatment. Chemosphere 2020; 244: 125420. https://doi.org/10.1016/j.chemosphere.2019.125420
[12] Lin Q, Donghui W, Jianlong W. Biodegradation of pyridine by Paracoccus sp. KT-5 immobilized on bamboo-based activated carbon. Bioresour Technol. 2010; 101: 5229-5234. https://doi.org/10.1016/j.biortech.2010.02.059
[13] Chen B, Yuan M, Qian L. Enhanced bioremediation of PAH-contaminated soil by immobilized bacteria with plant residue and biochar as carriers. J Soil Sediments. 2012; 12: 1350-1359. https://link.springer.com/article/10.1007/s11368-012-0554-5
[14] Shen Y, Li H, Zhu W, Ho SH, Yuan W, Chen J, Xie Y. Microalgal-BC 16. immobilized complex: a novel efficient biosorbent for cadmium removal from aqueous solution. Bioresour Technol. 2017; 244: 1031-1038. https://doi.org/10.1016/j.biortech.2017.08.085
[15] Peñalver Jl, linares-Fernández Jl, de Araujo Farías V, lópez-Ramón MV, Tassi M, Oliver FJ, Moreno-Castilla C, de Almodóvar JMR. Activated carbon cloth as support for mesenchymal stem cell growth and differentiation to osteocytes. Carbon, 2008; 47:3574-3577. https://doi.org/10.1016/j.carbon.2009.08.016
[16] Osmokrovic A, Jancic I, Vunduk J, Petrovic P, Milenkovic M, Obradovic B. Achieving high antimicrobial activity: Composite alginate hydrogel beads releasing activated charcoal with an immobilized active agent. Carb Pol. 2018; 196: 279-288. https://doi.org/10.1016/j.carbpol.2018.05.045
[17] ATCC Bacteriology Culture Guide. https://www.atcc.org/resources/culture-guides/bacteriology-culture-guide#preservation. Accessed February 1, 2025
[18] Tukey JW. Comparing individual means in the analysis of variance. Biometrics 1949; 5(2): 99–114. https://doi.org/10.2307/3001913
[19] Malik KA. A simplified liquid-drying method for the preservation of microorganisms sensitive to freezing and freeze-drying. J Microbiol Methods. 1990; 12: 125-132. https://doi.org/10.1016/0167-7012(90)90022-X
[20] Oluwatosin SO, Tai SL, Fagan-Endres MA. Sucrose, maltodextrin and inulin efficacy as cryoprotectant, preservative and prebiotic - towards a freeze dried Lactobacillus plantarum topical probiotic. Biotechnol Rep (Amst). 2021; 33: e00696. https://doi.org/10.1016/j.btre.2021.e00696
[21] Drvenica I, Stancic A, Kalusevic A, Markovic S, Dragisic Maksimovic J, Nedovic V, Bugarski B, Ilic V. Maltose-mediated, long-term stabilization of freeze- and spray-dried forms of bovine and porcine haemoglobin. J Serb Chem Soc. 2019; 84(10): 1105-1117. https://doi.org/10.2298/JSC190513067D
[22] Fan X, Shi Y, Li R, Yang R, Yang X, Hang F, Chen W. Preliminary study on the effect of pre-freezing methods on lyophilization quality and storage stability of probiotics. Dry Technol. 2024; 42(9): 1480-1492. https://doi.org/10.1080/07373937.2024.2361351
[23] Hartke A, Bouche S, Giard J-Ch, Benachour A, Boutibonnes Ph, Auffray Y. The lactic acid stress response of Lc. lactis subsp. lactis. Curr. Microbiol. 1996; 33: 194-199. https://link.springer.com/article/10.1007/s002849900099
[24] Kim WS, Dunn NW. Identification of a cold shock gene in lactic acid bacteria and the effect of cold shock on cryotolerance. Curr Microbiol. 1997; 35: 59-63. https://link.springer.com/article/10.1007/s002849900212
[25] Bâati L, Fabre-Gea C, Auriol D, Blanc PJ. Study of the cryotolerance of Lactobacillus acidophilus: effect of culture and freezing conditions on the viability and cellular protein levels. Int J Food Microbiol. 2000; 59 (3): 241-247. https://doi.org/10.1016/S0168-1605(00)00361-5