Effect of pretreatment, lyophilization parameters and different cryoprotectants on the efficiency of probiotic freeze-drying immobilization Original scientific paper
Main Article Content
Abstract
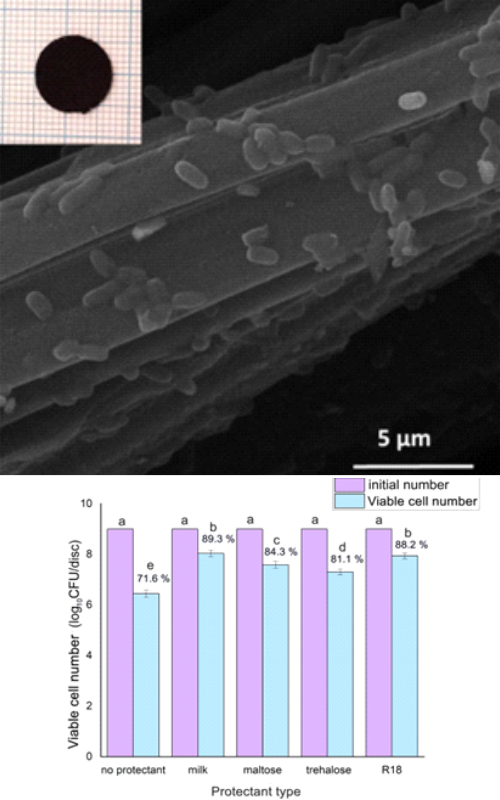
Lyophilization is an excellent process to increase the shelf life of food products or preserve probiotics. Living cells prefer mild conditions, and any deviation (vacuum, high or low temperatures) leads to cell damage. This paper examined the influence of different freeze-drying process parameters on the survival of probiotic Lactobacillus plantarum immobilized on the activated charcoal pad. In specific, the process included several phases in which different pretreatments, freezing temperatures, cryoprotectants and phase durations were investigated. Activation of L. plantarum in De Man, Rogosa and Sharpe (MRS) broth before freezing, resulted in the increased initial number of living cells, but also positively affected the cell survival after the lyophilization process. Freezing the culture in liquid nitrogen did not significantly affect cell viability after lyophilization compared to deep freezing at -80 °C, while incubation of the culture in a refrigerator for 2 h before lyophilization increased the probiotic viability. Also, the prolonged duration of lyophilization from 5 to 48 h had a slight impact on probiotic viability. The use of milk showed a significant increase in culture survival, while sucrose, maltose, and trehalose showed cryoprotectant ability, but significantly lower than that of milk. The best lyophilization protocol resulting in the highest viability of L. plantarum included the culture activation by MRS incubation, followed by either deep freezing in liquid nitrogen or by precooling for 2 h at 7 °C and then deep freezing at -80 °C in milk, and ending with lyophilization for 5 h.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
Funding data
-
Science Fund of the Republic of Serbia
Grant numbers Grant No 9802
References
[1] Malik KA. Survival and stability of microorganisms during freeze-drying. Cryobiology. 1988; 25(6): 517-518. https://doi.org/10.1016/0011-2240(88)90324-0
[2] Champagne CP, Gardner N., Brochu E., Beaulieu Y. The freeze-drying of lactic acid bacteria. Can Inst Food Technol J. 1991; 24: 118-128. https://doi.org/10.1016/S0315-5463(91)70034-5
[3] Hubálek Z. Protectants used in the cryopreservation of microorganisms. Cryobiology. 2003; 2003; 46: 205-229. https://doi.org/10.1016/S0011-2240(03)00046-4
[4] Alonso S. Novel Preservation Techniques for Microbial Cultures. in Ojha K, Tiwari B, eds. Novel Food Fermentation Technologies. Food Engineering Series. Springer; 2016: 8-33. https://doi.org/10.1007/978-3-319-42457-6_2
[5] Carvalho AS, Silva J, Ho P, Teixeira P, Malcata FX, Gibbs P. Relevant factors for the preparation of freeze-dried lactic acid bacteria. Int Dairy J. 2004; 14: 835-847. https://doi.org/10.1016/j.idairyj.2004.02.001
[6] Carvalho AS, Silva J, Ho P, Teixeira P, Malcata FX, Gibbs P. Survival of freeze-dried Lactobacillus plantarum and Lactobacillus rhamnosus during storage in the presence of protectants. Biotechnol Lett. 2002; 24: 1587-1591. https://link.springer.com/article/10.1023/A:1020301614728
[7] Morgan CA, Herman N, White PA, Vesey G. Preservation of micro-organisms by drying. J Microbiol Methods. 2006; 66: 183-193. https://doi.org/10.1016/j.mimet.2006.02.017
[8] Bouabidi ZB, El-Naas MH, Zhang Z. Immobilization of microbial cells for the biotreatment of wastewater. Environ Chem Lett. 2019; 17: 241-257. https://doi.org/10.1007/s10311-018-0795-7
[9] Wu P, Wang Z, Bhatnagar A, Jeyakumar P, Wang H, Wang Y, Li X, Microorganisms-carbonaceous materials immobilized complexes: Synthesis, adaptability and environmental applications. J Hazard Mater. 2021; 416: 125915. https://doi.org/10.1016/j.jhazmat.2021.125915
[10] Partovinia A, Rasekh B. Review of the immobilized microbial cell systems for bioremediation of petroleum hydrocarbons polluted environments. Crit Rev Environ Sci Technol. 2018; 48 (1): 1-38. https://doi.org/10.1080/10643389.2018.1439652
[11] Gong W, Fan Y, Xie B, Tang X, Guo T, Luo L, Liang H. Immobilizing Microcystis aeruginosa and powdered activated carbon for the anaerobic digestate effluent treatment. Chemosphere 2020; 244: 125420. https://doi.org/10.1016/j.chemosphere.2019.125420
[12] Lin Q, Donghui W, Jianlong W. Biodegradation of pyridine by Paracoccus sp. KT-5 immobilized on bamboo-based activated carbon. Bioresour Technol. 2010; 101: 5229-5234. https://doi.org/10.1016/j.biortech.2010.02.059
[13] Chen B, Yuan M, Qian L. Enhanced bioremediation of PAH-contaminated soil by immobilized bacteria with plant residue and biochar as carriers. J Soil Sediments. 2012; 12: 1350-1359. https://link.springer.com/article/10.1007/s11368-012-0554-5
[14] Shen Y, Li H, Zhu W, Ho SH, Yuan W, Chen J, Xie Y. Microalgal-BC 16. immobilized complex: a novel efficient biosorbent for cadmium removal from aqueous solution. Bioresour Technol. 2017; 244: 1031-1038. https://doi.org/10.1016/j.biortech.2017.08.085
[15] Peñalver Jl, linares-Fernández Jl, de Araujo Farías V, lópez-Ramón MV, Tassi M, Oliver FJ, Moreno-Castilla C, de Almodóvar JMR. Activated carbon cloth as support for mesenchymal stem cell growth and differentiation to osteocytes. Carbon, 2008; 47:3574-3577. https://doi.org/10.1016/j.carbon.2009.08.016
[16] Osmokrovic A, Jancic I, Vunduk J, Petrovic P, Milenkovic M, Obradovic B. Achieving high antimicrobial activity: Composite alginate hydrogel beads releasing activated charcoal with an immobilized active agent. Carb Pol. 2018; 196: 279-288. https://doi.org/10.1016/j.carbpol.2018.05.045
[17] ATCC Bacteriology Culture Guide. https://www.atcc.org/resources/culture-guides/bacteriology-culture-guide#preservation. Accessed February 1, 2025
[18] Tukey JW. Comparing individual means in the analysis of variance. Biometrics 1949; 5(2): 99–114. https://doi.org/10.2307/3001913
[19] Malik KA. A simplified liquid-drying method for the preservation of microorganisms sensitive to freezing and freeze-drying. J Microbiol Methods. 1990; 12: 125-132. https://doi.org/10.1016/0167-7012(90)90022-X
[20] Oluwatosin SO, Tai SL, Fagan-Endres MA. Sucrose, maltodextrin and inulin efficacy as cryoprotectant, preservative and prebiotic - towards a freeze dried Lactobacillus plantarum topical probiotic. Biotechnol Rep (Amst). 2021; 33: e00696. https://doi.org/10.1016/j.btre.2021.e00696
[21] Drvenica I, Stancic A, Kalusevic A, Markovic S, Dragisic Maksimovic J, Nedovic V, Bugarski B, Ilic V. Maltose-mediated, long-term stabilization of freeze- and spray-dried forms of bovine and porcine haemoglobin. J Serb Chem Soc. 2019; 84(10): 1105-1117. https://doi.org/10.2298/JSC190513067D
[22] Fan X, Shi Y, Li R, Yang R, Yang X, Hang F, Chen W. Preliminary study on the effect of pre-freezing methods on lyophilization quality and storage stability of probiotics. Dry Technol. 2024; 42(9): 1480-1492. https://doi.org/10.1080/07373937.2024.2361351
[23] Hartke A, Bouche S, Giard J-Ch, Benachour A, Boutibonnes Ph, Auffray Y. The lactic acid stress response of Lc. lactis subsp. lactis. Curr. Microbiol. 1996; 33: 194-199. https://link.springer.com/article/10.1007/s002849900099
[24] Kim WS, Dunn NW. Identification of a cold shock gene in lactic acid bacteria and the effect of cold shock on cryotolerance. Curr Microbiol. 1997; 35: 59-63. https://link.springer.com/article/10.1007/s002849900212
[25] Bâati L, Fabre-Gea C, Auriol D, Blanc PJ. Study of the cryotolerance of Lactobacillus acidophilus: effect of culture and freezing conditions on the viability and cellular protein levels. Int J Food Microbiol. 2000; 59 (3): 241-247. https://doi.org/10.1016/S0168-1605(00)00361-5