3D biomaterials produced by near-field electrospinning and melt electrowriting Mini-review Paper
Glavni sadržaj članka
Apstrakt
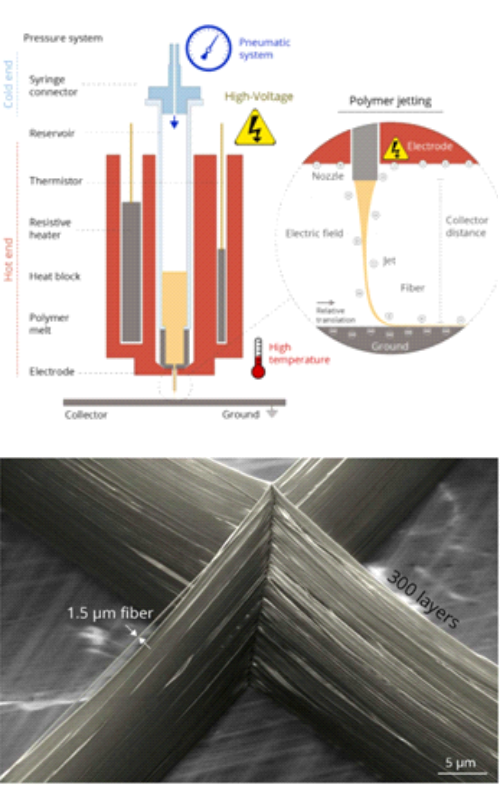
Near-field electrospinning and melt electrowriting, are attractive techniques that can be used to produce polymeric nano- or microfibres and build three-dimensional (3D) shapes that can be used in biotechnology and biomedicine. Preferred patterns can be designed due to the possibility to define nozzle and collector movements. Opposite to conventional electrospinning, near-field electrospinning enables formation of very fine fibres assembled in structures with much larger pore sizes, tailored according to the requirements of cells, which makes such scaffolds highly interesting for cell culture, tissue engineering applications and similar biomedical and biotechnological applications. In addition, this technique is relatively simple, reproducible and inexpensive. Melt electrowriting can be used to draw microfibres from a solution or a melt through an electrostatic field allowing precise deposition with high accuracy, leading to highly porous scaffolds that facilitate homogeneous cell distribution. This review provides an overview of new theoretical and experimental findings related to near-field electrospinning and melt electrowriting for applications in biotechnology and biomedicine, such as printing scaffolds for tissue engineering and cell culture, producing wound dressings, and others. Near-field electrospinning and melt electrowriting processes are briefly explained, and the most relevant polymers for biomedical applications are presented. Finally, recent challenges and suggestions for future research directions are given.
Detalji članka
Broj časopisa
Rubrika

Ovaj rad je pod Creative Commons Autorstvo 4.0 Internacionalna licenca.
Kada je rukopis prihvaćen za objavlјivanje, autori prenose autorska prava na izdavača. U slučaju da rukopis ne bude prihvaćen za štampu u časopisu, autori zadržavaju sva prava.
Na izdavača se prenose sledeća prava na rukopis, uklјučujući i dodatne materijale, i sve delove, izvode ili elemente rukopisa:
- pravo da reprodukuje i distribuira rukopis u štampanom obliku, uklјučujući i štampanje na zahtev;
- pravo na štampanje probnih primeraka, reprint i specijalnih izdanja rukopisa;
- pravo da rukopis prevede na druge jezike;
- pravo da rukopis reprodukuje koristeći fotomehanička ili slična sredstva, uklјučujući, ali ne ograničavajući se na fotokopiranje, i pravo da distribuira ove kopije;
- pravo da rukopis reprodukuje i distribuira elektronski ili optički koristeći sve nosioce podataka ili medija za pohranjivanje, a naročito u mašinski čitlјivoj/digitalizovanoj formi na nosačima podataka kao što su hard disk, CD-ROM, DVD, Blu-ray Disc (BD), mini disk, trake sa podacima, i pravo da reprodukuje i distribuira rukopis sa tih prenosnika podataka;
- pravo da sačuva rukopis u bazama podataka, uklјučujući i onlajn baze podataka, kao i pravo prenosa rukopisa u svim tehničkim sistemima i režimima;
- pravo da rukopis učini dostupnim javnosti ili zatvorenim grupama korisnika na osnovu pojedinačnih zahteva za upotrebu na monitoru ili drugim čitačima (uklјučujući i čitače elektonskih knjiga), i u štampanoj formi za korisnike, bilo putem interneta, onlajn servisa, ili putem internih ili eksternih mreža.
Kako citirati
Reference
[1] Smith LA, Ma PX. Nano-fibrous scaffolds for tissue engineering. Colloids Surf B. 2004; 3: 125-131. https://doi.org/10.1016/j.colsurfb.2003.12.004
[2] Jun ID, Han HS, Edwards JR, Jeon HJ. Electrospun fibrous scaffolds for Tissue engineering: viewpoints on architecture and fabrication. Int J Mol Sci. 2018; 19: 745. https://doi.org/10.3390/ijms19030745
[3] Rahman M, Dip TM, Nur MG, Padhye R, Houshyar S. Fabrication of Silk Fibroin-Derived Fibrous Scaffold for Biomedical Frontiers. Macromol Mater Eng. 2024; 309: 2300422. https://doi.org/10.1002/mame.202300422
[4] Ji DX, Lin YG, Guo XY, Ramasubramanian B, Wang RW, Radacsi N, Jose R, Qin XH, Ramakrishna S. Electrospinning of nanofibres. Nat Rev Methods Primers. 2024; 4: 1. https://doi.org/10.1038/s43586-023-00278-z
[5] Maran BAV, Jeyachandran S, Kimura M. A review on the electrospinning of polymer nanofibers and Its Biomedical Applications. J Comp Sci. 2024; 8: 32. https://doi.org/10.3390/jcs8010032
[6] Wang CL, Su YJ, Xie JW. Advances in Electrospun Nanofibers: Versatile materials and diverse biomedical applications. Acc Mater Res. 2024; 5: 987-999. https://doi.org/10.1021/accountsmr.4c00145
[7] Teyeb C, Grothe T, Dotter M, Kola I, Ehrmann A. Homogeneity of physical properties of electrospun gelatin nanofiber mats. Sust Green Mater. 2024; 1-14, https://doi.org/10.1080/29965292.2024.2404716
[8] Morina E, Dotter M, Döpke C, Kola I, Spahiu T, Ehrmann A. Homogeneity of Needleless Electrospun Nanofiber Mats. Nanomaterials. 2023; 13: 2507. https://doi.org/10.3390/nano13182507
[9] Robinson AJ, Pérez-Nava A, Ali SC, González-Campos JB, Holloway JL. Comparative analysis of fiber alignment methods in electrospinning. Matter. 2021; 4: 821-844. https://doi.org/10.1016/j.matt.2020.12.022
[10] Blachowicz T, Malczyk M, Kola I, Ehrmann A. Textiles for Very Cold Environments. Processes. 2024; 12: 927. https://doi.org/10.3390/pr12050927
[11] Blachowicz T, Mpofu NS, Ehrmann A. Measuring Physical and Chemical Properties of Single Nanofibers for Energy Applications—Possibilities and Limits. Nanoenergy Adv. 2024; 4: 300-317. https://doi.org/10.3390/nanoenergyadv4040018
[12] Blachowicz T, Ehrmann A. Methods and Engineering of Electrospinning. In: Das R, ed. Electrospun Nanofibrous Technology for Clean Water Production. Nanostructure Science and Technology. Springer, Singapore; 2023:7-35. https://doi.org/10.1007/978-981-99-5483-4_2
[13] Hellert C, Storck JL, Grothe T, Kaltschmidt B, Hütten A, Ehrmann A. Positioning and Aligning Electrospun PAN Fibers by Conductive and Dielectric Substrate Patterns. Macromol Symp. 2021; 395: 2000213. https://doi.org/10.1002/masy.202000213
[14] Storck JL, Grothe T, Mamun A, Sabantina A, Klöcker M, Blachowicz T, Ehrmann A. Orientation of Electrospun Magnetic Nanofibers Near Conductive Areas. Materials. 2020; 13: 47. https://doi.org/10.3390/ma13010047
[15] Storck JL, Brockhagen B, Grothe T, Sabantina L, Kaltschmidt B, Tuvshinbayar K, Braun L, Tanzli E, Hütten A, Ehrmann A. Stabilization and Carbonization of PAN Nanofiber Mats Electrospun on Metal Substrates. C 2021; 7: 12. https://doi.org/10.3390/c7010012
[16] Kameoka J, Orth R, Yang Y, Czaplewski D, Mathers R, Coates GW, Craighead H. A scanning tip electrospinning source for deposition of oriented nanofibres. Nanotechnology. 2003; 14: 1124. https://doi.org/10.1088/0957-4484/14/10/310
[17] Sun D, Chang C, Li S, Lin L. Near-field electrospinning. Nano Lett. 2006; 6: 839-842. https://doi.org/10.1021/nl0602701
[18] Mpofu NS, Blachowicz T, Ehrmann A, Ehrmann G. Wearable Electrospun Nanofibrous Sensors for Health Monitoring. Micro. 2024; 4: 798-822. https://doi.org/10.3390/micro4040049
[19] Mieszczanek P, Robinson TM, Dalton PD, Hutmacher DW. Convergence of machine vision and melt electrowriting. Adv Mater. 2021; 33: 2100519. https://doi.org/10.1002/adma.202100519
[20] Bisht G, Nesterenko S, Kulinsky L, Madou M. A computer-controlled near-field electrospinning setup and its graphic user interface for precision patterning of functional nanofibers on 2D and 3D substrates. SLAS Technology. 2012; 17: 302-308. https://doi.org/10.1177/2211068212446372
[21] Khodabandeh AR, Yousefi AA, Vasheghani-Farahani E. The effect of process variables on near-field electrospinning of polycaprolactone studied by response surface methodology. Iran Polym J. 2024; 33: 1569-1581. https://doi.org/10.1007/s13726-024-01339-0
[22] Yang Y, Jia Z, Liu J, Li Q, Hou L, Wang L, Guan Z. Effect of electric field distribution uniformity on electrospinning. J Appl Phys. 2008; 103: 104307. https://doi.org/10.1063/1.2924439
[23] Hekmati A, Rashidi A, Ghazisaeidi R, Drean JY. Effect of needle length, electrospinning distance, and solution concentration on morphological properties of polyamide-6 electrospun nanowebs. Text Res J. 2013; 83: 1452-1466. https://doi.org/10.1177/0040517512471746
[24] Reizabal A, Kangur T, Saiz PG, Menke S, Moser C, Brugger J, Menke S, Moser C, Brugger J, Dalton PD, Luposchainsky S. MEWron: An open-source melt electrowriting platform. Addit Manuf. 2023; 71: 103604. https://doi.org/10.1016/j.addma.2023.103604
[25] Peng ZL, Wang MJ, Lv H, Zhang JY, Li YN, Wu JY, Zhang SL, Wang F, Zhang GM, Zhu XY, Xu L, Lan HB. Electric field-driven microscale 3D printing of flexible thin-walled tubular mesh structures of molten polymers. Mater Des. 2023; 225: 111433. https://doi.org/10.1016/j.matdes.2022.111433
[26] Reizabal A, Devlin BL, Paxton NC, Saiz PG, Liashenko I, Luposchainsky S, Woodruff MA, Lanceros-Mendez S, Dalton PD. Melt electrowriting of nylon-12 microfibers with an open-source 3D printer. Macromol Rapid Comm. 2023; 44: 2300424. https://doi.org/10.1002/marc.202300424
[27] Vazquez-Armendariz J, Tejeda-Alejandre R, Bahhur A, Rodriguez CA, Dean D. Meltelectrowriting of polycaprolactone thin membranes with high porosity. Proc CIRP. 2022; 110: 282-286. https://doi.org/10.1016/j.procir.2022.06.051
[28] O’Neill KL, Dalton PD. A decade of melt electrowriting. Small Methods. 2023; 7: 2201589. https://doi.org/10.1002/smtd.202201589
[29] Ding HZ, Cao K, Zhang FC, Boettcher W, Chang RC. A fundamental study of charge effects on melt electrowritten polymer fibers. Mater Des. 2019; 178: 107857. https://doi.org/10.1016/j.matdes.2019.107857
[30] King III WE, Bowlin GL. Near-field electrospinning and melt electrowriting of biomedical polymers – progress and limitations. Polymers. 2021; 13: 1097. https://doi.org/10.3390/polym13071097
[31] Kade JC, Dalton PD. Polymers for melt electrowriting. Adv Healthcare Mater. 2020; 10: 2001232. https://doi.org/10.1002/adhm.202001232
[32] Saiz PG, Reizabal A, Vilas-Vilela JL, Dalton PD, Lanceros-Mendez S. Materials and strategies to enhance melt electrowriting potential. Adv Mater. 2024; 36: 2312084. https://doi.org/10.1002/adma.202312084
[33] Loewner S, Heene S, Baroth T, Heymann H, Cholewa F, Blume H, Blume C. Recent advances in melt electro writing for tissue engineering for 3D printing of microporous scaffolds for tissue engineering. Front Bioeng Biotechnol. 2022; 10: 896719. https://doi.org/10.3389/fbioe.2022.896719
[34] Yan FF, Chen HP, Zheng LL, Chen WH, Liu YY, Hu QX. The controllable PVA-Chitosan fiber prepared by the near-field electro spinning for tissue engineering. Adv J Food Sci Technol. 2013; 5: 1073-1078. https://maxwellsci.com/print/ajfst/v5-1073-1078.pdf
[35] King III WE, Bowlin GL. Near-field electrospinning of polydioxanone small diameter vascular graft scaffolds. J Mech Behav Biomed Mater. 2022; 130: 105207. https://doi.org/10.1016/j.jmbbm.2022.105207
[36] King III WE, Bowlin GL. Mechanical characterization and neutrophil NETs response of a novel hybrid geometry polydioxanone near-field electrospun scaffold. Biomed Mater. 2021; 16: 065002. https://doi.org/10.1088/1748-605X/ac1e43
[37] Snyder AE, Sandridge JK, Nordmoe AE, Main EN, Bowlin GL. Fabrication and mechanical characterization of near field electrospun bioresorbable vascular grafts with fibrous architecture mimicking the arterial extracellular matrix. J Bioact Compat Pol. 2024; 39: 455-466. https://doi.org/10.1177/08839115241262038
[38] Qavi I, Tan GZ. Near-field electrospinning polycaprolactone microfibers to mimic arteriole-capillary–venule structure. Progr Biomater. 2021; 10: 223-233. https://doi.org/10.1007/s40204-021-00165-4
[39] Davis ZG, Hussain AF, Fisher MB. Processing variables of direct-write, near-field electrospinning impact size and morphology of gelatin fibers. Biomed Mater. 2021; 16: 045017. https://doi.org/10.1088/1748-605X/abf88b
[40] Wehlage D, Blattner H, Sabantina L, Böttjer R, Grothe T, Rattenholl A, Gudermann F, Lütkemeyer D, Ehrmann A. Sterilization of PAN/gelatine nanofibrous mats for cell growth. Tekstilec. 2019; 62: 78-88. https://doi.org/10.14502/Tekstilec2019.62.78-88
[41] Li DF, Lin DS, Li Y, Xu SK; Cao QY, Zhou WY. Preparation and characterization of novel multifunctional wound dressing by near-field direct-writing electrospinning and its appliation. Polymers. 2024; 16: 1573. https://doi.org/10.3390/polym16111573
[42] Mai ZR, Liu QL, Bian YS, Wang P, Fu XW, Lin DS, Kong NZ, Huang YQ, Zeng ZJ, Li DF, Zheng WX, Xia YJ, Zhou WY. PCL/collagen/UA composite biomedical dressing with ordered microfiberous structure fabricated by a 3D near-field electrospinning process. Polymers. 2022; 15: 223. https://doi.org/10.3390/polym15010223
[43] Fuchs A, Youssef A, Seher A, Hartmann S, Brands RC, Müller-Richter UDA, Kübler AC, Linz C. A new multilayered membrane for tissue engineering of oral hard- and soft tissue by means of melt electrospinning writing and film casting – An in vitro study. J Craniomaxillofac Surg. 2019; 47: 695-703. https://doi.org/10.1016/j.jcms.2019.01.043
[44] Eichholz KF, Hoey DA. Mediating human stem cell behaviour via defined fibrous architectures by melt electrospinning writing. Acta Biomater. 2018; 75: 140-151. https://doi.org/10.1016/j.actbio.2018.05.048
[45] Black C, Kanczler JM, de Andrés MC, White LJ, Savi FM, Bas O, Saifzadeh S, Henkel J, Zannettino A, Gronthos S, Woodruff MA, Hutmacher D W, Oreffo ROC. Characterisation and evaluation of the regenerative capacity of Stro-4+ enriched bone marrow mesenchymal stromal cells using bovine extracellular matrix hydrogel and a novel biocompatible melt electro-written medical-grade polycaprolactone scaffold. Biomaterials. 2020; 247: 119998. https://doi.org/10.1016/j.biomaterials.2020.119998
[46] Chen Z J, Liu YB, Huang J, Wang H, Hao M, Hu XD, Qian XM, Fan JT, Yang HJ, Yang B. Enhanced in vitro biocompatible polycaprolactone/nano-hydroxyapatite scaffolds with near-field direct-writing melt electrospinning technology. J Funct Biomater. 2022; 13: 161. https://doi.org/10.3390/jfb13040161
[47] Chen ZJ, Liu YB, Huang J, Hao M, Hu, XD, Qian XM, Fan JT, Yang HJ, Yang B. Influences of process parameters of near-field direct-writing melt electrospinning on performances of polycaprolactone/nano-hydroxyapatite scaffolds. Polymers. 2022; 14: 3404. https://doi.org/10.3390/polym14163404
[48] Gwiazda M, Kumar S, Swieszkowski W, Ivanovski S, Vaquett C. The effect of melt electrospun writing fiber orientation onto cellular organization and mechanical properties for application in Anterior Cruciate Ligament tissue engineering. J Mech Behav Biomed Mater. 2020; 104: 103631. https://doi.org/10.1016/j.jmbbm.2020.103631
[49] Ma SS, Zheng SY, Li D, Hu WH, Wang LM. Melt Electrowriting Combined with Fused Deposition Modeling Printing for the Fabrication of Three-Dimensional Biomimetic Scaffolds for Osteotendinous Junction Regeneration. Int J Nanomed. 2024; 19: 3275-3293. https://doi.org/10.2147/IJN.S449952
[50] Meng J, Boschetto F, Yagi S, Marin E, Adachi T, Chen XF, Pezzotti G, Sakurai S, Yamane H, Xu HZ. Design and manufacturing of 3D high-precision micro-fibrous poly (l-lactic acid) scaffold using melt electrowriting technique for bone tissue engineering. Mater Des. 2021; 210: 110063. https://doi.org/10.1016/j.matdes.2021.110063
[51] Shahverdi M, Seifi S, Akbari A, Mohammadi K, Shamloo A, Movahhedy MR. Melt electrowriting of PLA, PCL, and composite PLA/PCL scaffolds for tissue engineering application. Sci Rep. 2022; 12: 19935. https://doi.org/10.1038/s41598-022-24275-6
[52] Soukarie D, Nocete L, Bittner AM, Santiago I. DNA data storage in electrospun and melt-electrowritten composite nucleic acid-polymer fibers. Mater Bio Today. 2024; 24: 100900. https://doi.org/10.1016/j.mtbio.2023.100900
[53] Qiu ZN, Wang YT, Kasimu A, Li DC, He JK. Functionalized alginate-based bioinks for microscale electrohydrodynamic bioprinting of living tissue constructs with improved cellular spreading and alignment. Bio-Des Manufact. 2023; 6: 136-149. https://doi.org/10.1007/s42242-022-00225-z
[54] Ross MT, Kilian D, Lode A, Ren JY, Allenby MC, Gelinsky M, Woodruff MA. Using melt-electrowritten microfibres for tailoring scaffold mechanics of 3D bioprinted chondrocyte-laden constructs. Bioprinting. 2021; 23: e00158. https://doi.org/10.1016/j.bprint.2021.e00158
[55] Paxton NC, Lanaro M, Bo A, Crooks N, Ross MT, Green N, Tetsworth K, Allenby MC, Gu YT, Wong CS, Powell SK, Woodruff MA. Design tools for patient specific and highly controlled melt electrowritten scaffolds. J Mech Behav Biomed Mater. 2020; 105: 103695. https://doi.org/10.1016/j.jmbbm.2020.103695