Valorizacija sojine sačme za proizvodnju visokoproteinske stočne hrane i proizvoda sa dodatom vrednošću korišćenjem novog soja Aureobasidium pullulans Naučni rad
Glavni sadržaj članka
Apstrakt
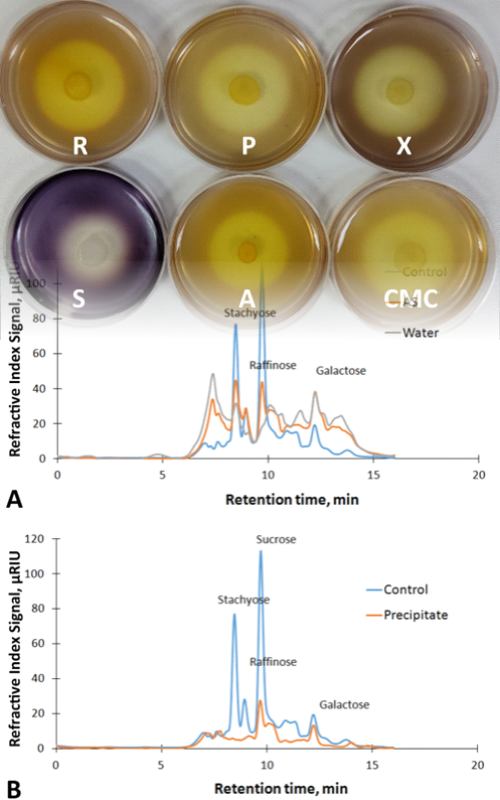
Sojina sačma je nusproizvod koji nastaje nakon ekstrakcije ulja iz zrna soje. S obzirom na to da je bogata visokokvalitetnim proteinima, sojina sačma se koristi kao dodatak za stočnu hranu. Međutim, bogata je takođe i antinutritivnim faktorima i nesvarljivim komponentama, među kojima je posebna pažnja usmerena na galaktooligosaharide, zbog nedostatka α-galaktozidaze kod monogastričnih životinja. Osnovni cilj ovog istraživanja bio je da se odabere pogodan soj crne gljivice nalik kvascu (Aureobasidium spp.) među deset prirodnih izolata iz grožđa, koji bi tokom fermentacije sojine sačme dao proizvod sa velikim sadržajem proteina i malim sadržajem oligosaharida. Sa tim ciljem odabran je izolat P8 koji je pokazao najveću aktivnost α-galaktozidaze od 0,89 U cm-3. Odabrani soj je identifikovan kao A. pullulans P8. Maksimalni prinos sirovih proteina u fermentisanoj sojinoj sačmi (61 % računato na suvu materiju) i najmanji sadržaj galaktooligosaharida dobijeni su nakon 3 dana inkubacije na 30 °C potopnom fermentacijom pri sadržaju 10 % suve materije sojine sačme. Fermentacijom na čvrstom supstratu dobijeno je ~58 % sirovih proteina nakon 7 dana inkubacije na 30 °C pri sadržaju suve materije od 30 %. U supernatantu dobijenom nakon potopne fermentacije izmerene su aktivnosti ekstracelularnih enzima (celulaze, pektinaze, amilaze, ksilanaze i α-galaktozidaze), što ukazuje na potencijal ovog pomoćnog proizvoda za hidrolizu različitih lignoceluloznih supstrata.
Detalji članka
Broj časopisa
Rubrika

Ovaj rad je pod Creative Commons Autorstvo 4.0 Internacionalna licenca.
Kada je rukopis prihvaćen za objavlјivanje, autori prenose autorska prava na izdavača. U slučaju da rukopis ne bude prihvaćen za štampu u časopisu, autori zadržavaju sva prava.
Na izdavača se prenose sledeća prava na rukopis, uklјučujući i dodatne materijale, i sve delove, izvode ili elemente rukopisa:
- pravo da reprodukuje i distribuira rukopis u štampanom obliku, uklјučujući i štampanje na zahtev;
- pravo na štampanje probnih primeraka, reprint i specijalnih izdanja rukopisa;
- pravo da rukopis prevede na druge jezike;
- pravo da rukopis reprodukuje koristeći fotomehanička ili slična sredstva, uklјučujući, ali ne ograničavajući se na fotokopiranje, i pravo da distribuira ove kopije;
- pravo da rukopis reprodukuje i distribuira elektronski ili optički koristeći sve nosioce podataka ili medija za pohranjivanje, a naročito u mašinski čitlјivoj/digitalizovanoj formi na nosačima podataka kao što su hard disk, CD-ROM, DVD, Blu-ray Disc (BD), mini disk, trake sa podacima, i pravo da reprodukuje i distribuira rukopis sa tih prenosnika podataka;
- pravo da sačuva rukopis u bazama podataka, uklјučujući i onlajn baze podataka, kao i pravo prenosa rukopisa u svim tehničkim sistemima i režimima;
- pravo da rukopis učini dostupnim javnosti ili zatvorenim grupama korisnika na osnovu pojedinačnih zahteva za upotrebu na monitoru ili drugim čitačima (uklјučujući i čitače elektonskih knjiga), i u štampanoj formi za korisnike, bilo putem interneta, onlajn servisa, ili putem internih ili eksternih mreža.
Kako citirati
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-65/2024-03/200135;451-03-66/2024-03/200287 -
Innovation Fund of the Republic of Serbia
Grant numbers Voucher number 1076
Reference
[1] ReportLinker. https://www.reportlinker.com/clp/global/3685. Accessed June 26, 2025.
[2] Shiu YL, Wong SL, Guei WC, Shin YC, Liu CH. Increase in the plant protein ratio in the diet of white shrimp, Litopenaeus vannamei (Boone), using Bacillus subtilis E20-fermented soybean meal as a replacement. Aquac Res. 2015; 46(2): 382-394. https://doi.org/10.1111/are.12186
[3] Karr-Lilienthal LK, Kadzere CT, Grieshop CM, Fahey GC. Chemical and nutritional properties of soybean carbohydrates as related to nonruminants. Livest Prod Sci. 2005; 97(1): 1-12. https://doi.org/10.1016/j.livprodsci.2005.01.015
[4] Abdel-Raheem SM, Mohammed ESY, Mahmoud RE, Gamal MFE, Nada HS, El-Ghareeb WR, Marzok M, Meligy AMA, Abdulmohsen M, Ismail H, Ibrahim D, Kishawy ATY. Double-Fermented Soybean Meal Totally Replaces Soybean Meal in Broiler Rations with Favorable Impact on Performance, Digestibility, Amino Acids Transporters and Meat Nutritional Value. Animals. 2023; 13(6): 1030. https://doi.org/10.3390/ani13061030
[5] Middelbos IS, Fahey GC. Soybean Carbohydrates. In: Johnson LA, White PJ, Galloway R, eds. Soybeans Chemistry, Production, Processing, and Utilization. Urbana, IL: AOCS Press; 2008. p. 269-296. https://doi.org/10.1016/B978-1-893997-64-6.50012-3
[6] Anisha GS. Microbial α-galactosidases: Efficient biocatalysts for bioprocess technology. Bioresour Technol 2022; 344: 126293. https://doi.org/10.1016/j.biortech.2021.126293
[7] Celi P, Cowieson AJ, Fru-Nji F, Steinert RE, Kluenter A-M, Verlhac V. Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal production. Anim Feed Sci Technol. 2017; 234: 88-100. https://doi.org/10.1016/j.anifeedsci.2017.09.012
[8] Zhu J, Gao M, Zhang R, et al. Effects of soybean meal fermented by L. plantarum, B. subtilis and S. cerevisieae on growth, immune function and intestinal morphology in weaned piglets. Microb Cell Fact. 2017; 16(1): 191. https://doi.org/10.1186/s12934-017-0809-3
[9] Shang QH, Ma XK, Li M, Zhang LH, Hu JX, Piao XS. Effects of α-galactosidase supplementation on nutrient digestibility, growth performance, intestinal morphology and digestive enzyme activities in weaned piglets. Anim Feed Sci Technol. 2018; 236: 48-56. https://doi.org/10.1016/j.anifeedsci.2017.11.008
[10] Milić MD, Buntić A V., Mihajlovski KR, Ilić N V., Davidović SZ, Dimitrijević-Branković SI. The development of a combined enzymatic and microbial fermentation as a viable technology for the spent coffee ground full utilization. Biomass Convers Biorefinery. 2023; 13(8): 6747-6759. https://doi.org/10.1007/s13399-021-01605-8
[11] Jakobsen G V., Jensen BB, Bach Knudsen KE, Canibe N. Impact of fermentation and addition of non-starch polysaccharide-degrading enzymes on microbial population and on digestibility of dried distillers grains with solubles in pigs. Livest Sci. 2015; 178: 216-227. https://doi.org/10.1016/j.livsci.2015.05.028
[12] Senanayake D, Torley PJ, Chandrapala J, Terefe NS. Microbial Fermentation for Improving the Sensory, Nutritional and Functional Attributes of Legumes. Fermentation. 2023;.9(7): 635. https://doi.org/10.3390/fermentation9070635
[13] Tang J, Li W, Zhou Q, et al. Effect of heating, microbial fermentation, and enzymatic hydrolysis of soybean meal on growth performance, nutrient digestibility, and intestinal microbiota of weaned piglets. J Anim Sci. 2023; 101: skad384. https://doi.org/10.1093/jas/skad384
[14] Xue J, Wu J, Ji Y, et al. Effect of microbial fermentation on the quality of soybean meal. Int J Food Sci Technol. 2024; 59(1): 72-83. https://doi.org/10.1111/ijfs.16817
[15] Baker KM, Liu Y, Stein HH. Nutritional value of soybean meal produced from high protein, low oligosaccharide, or conventional varieties of soybeans and fed to weanling pigs. Anim Feed Sci Technol. 2014; 188: 64-73. https://doi.org/10.1016/j.anifeedsci.2013.10.018
[16] Loman A Al, Ju LK. Soybean carbohydrate as fermentation feedstock for production of biofuels and value-added chemicals. Process Biochem. 2016; 51(8): 1046-1057. https://doi.org/10.1016/j.procbio.2016.04.011
[17] Deng Z, Duarte ME, Kim SY, Hwang Y, Kim SW. Comparative effects of soy protein concentrate, enzyme-treated soybean meal, and fermented soybean meal replacing animal protein supplements in feeds on growth performance and intestinal health of nursery pigs. J Anim Sci Biotechnol. 2023; 14(1): 89. https://doi.org/10.1186/s40104-023-00888-3
[18] Chen S, Zheng H, Gao J, Song H, Bai W. High-level production of pullulan and its biosynthesis regulation in Aureobasidium pullulans BL06. Front Bioeng Biotechnol. 2023; 11. https://doi.org/10.3389/fbioe.2023.1131875
[19] Cruz-Santos MM, Antunes FAF, Arruda GL, Shibukawa VP, Prado, CA, Ortiz-Silos N, Castro-Alonso MJ, Marcelino PRF, Santos JC. Production and applications of pullulan from lignocellulosic biomass: Challenges and perspectives. Bioresour Technol. 2023; 385: 129460. https://doi.org/10.1016/j.biortech.2023.129460
[20] Thirumavalavan K, Manikkadan TR, Dhanasekar R. Pullulan production from coconut by-products by Aureobasidium pullulans. African J Biotechnol. 2009; 8(2): 254-258
[21] Wang P, Jia S-L, Liu G-L, Chi Z, Chi Z-M. Aureobasidium spp. and their applications in biotechnology. Process Biochem. 2022; 116: 72-83. https://doi.org/10.1016/j.procbio.2022.03.006
[22] Di Francesco A, Sciubba L, Bencivenni M, Marzadori C, Baraldi E. Application of Aureobasidium pullulans in iron‐poor soil. Can the production of siderophores improve iron bioavailability and yeast antagonistic activity? Ann Appl Biol. 2022; 180(3): 398-406. https://doi.org/10.1111/aab.12742
[23] Rensink S, van Nieuwenhuijzen EJ, Sailer MF, Struck C, Wösten HAB. Use of Aureobasidium in a sustainable economy. Appl Microbiol Biotechnol. 2024; 108(1): 202. https://doi.org/10.1007/s00253-024-13025-5
[24] Carević M, Banjanac K, Ćorović M, et al. Selection of lactic acid bacteria strain for simultaneous production of α- and β-galactosidases. Zast Mater. 2016; 57(2): 265-273. https://doi.org/10.5937/zasmat1602265c
[25] Miller GL. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal Chem. 1959; 31(3): 426-428. https://doi.org/10.1021/ac60147a030
[26] Ilić N, Davidović S, Milić M, et al. Valorization of lignocellulosic wastes for extracellular enzyme production by novel Basidiomycetes: screening, hydrolysis, and bioethanol production. Biomass Convers Biorefinery. 2022; 13: 17175-17186. https://doi.org/10.1007/s13399-021-02145-x
[27] ISO 5983-1:2005; Animal feeding stuffs: Determination of nitrogen content and calculation of crude protein content. Part 1: Kjeldahl method, International Standard Organization. Technical Committee: Geneva, Switzerland, 2005; ISO/TC 34/SC 10. https://www.iso.org/standard/39145.html
[28] Thermo Fisher Scientific. Chromeleon (Version 7.2) [Computer Software]. Thermo Fisher Scientific, 2018. https://www.thermofisher.com/order/catalog/product/CHROMELEON7
[29] OriginLab Corporation. OriginPro (Version 9.0) [Computer Software]. OriginLab Corporation, 2012. https://www.originlab.com/
[30] van Nieuwenhuijzen EJ. Aureobasidium. Encycl Food Microbiol Second Ed 2014; 1: 105-109. https://doi.org/10.1016/B978-0-12-384730-0.00017-3
[31] Bankeeree W, Lotrakul P, Prasongsuk S, Kim SW, Punnapayak H. Enhanced Production of Cellulase-Free Thermoactive Xylanase Using Corncob by a Black Yeast, Aureobasidium pullulans CBS 135684. Korean Chem Eng Res. 2016; 54(6): 822-829. https://doi.org/10.9713/kcer.2016.54.6.822
[32] Mulay YR, Deopurkar RL. Production of amylase from indigenously isolated strain of Aureobasidium Pullulans and its hyper producing mutant. J Microbiol Biotechnol Food Sci. 2017; 7(3): 287-293. https://doi.org/10.15414/jmbfs.2017/18.7.3.287-293
[33] Zajc J, Černoša A, Francesco A Di, et al. Fungal Genomics & Biology Characterization of Aureobasidium pullulans Isolates Selected as Biocontrol Agents Against Fruit Decay Pathogens. Fungal Genom Biol. 2020; 10(1): 163. https://doi.org/10.35248/2165-8056.20.10.163
[34] Leite RSR, Bocchini DA, Martins EDS, Silva D, Gomes E, Da Silva R. Production of cellulolytic and hemicellulolytic enzymes from Aureobasidium pulluans on solid state fermentation. Appl Biochem Biotechnol. 2007; 137-140(1-12): 281-288. https://doi.org/10.1007/s12010-007-9058-y
[35] Thakur M, Hurburgh CR. Quality of US soybean meal compared to the quality of soybean meal from other origins. JAOCS, J Am Oil Chem Soc. 2007; 84(9): 835-843. https://doi.org/10.1007/s11746-007-1107-8
[36] Badr-Eldin SM, El-Tayeb OM, El-Masry HG, Mohamad FHA, El-Rahman OAA. Polysaccharide production by Aureobasidium pullulans: factors affecting polysaccharide formation. World J Microbiol Biotechnol. 1994; 10(4): 423-426. https://doi.org/10.1007/BF00144465
[37] Lotrakul P, Unhapattaratitikul P, Seelanan T, Prasongsuk S, Punnapayak H. An aubasidan-like β-glucan produced by Aureobasidium pullulans in Thailand. ScienceAsia. 2013; 39(4): 363. https://doi.org/10.2306/scienceasia1513-1874.2013.39.363
[38] Zhang Y, Ishikawa M, Koshio S, et al. Optimization of soybean meal fermentation for aqua-feed with bacillus subtilis natto using the response surface methodology. Fermentation. 2021; 7(4): 306. https://doi.org/10.3390/fermentation7040306
[39] Ilić N, Milić M, Beluhan S, Dimitrijević-Branković S. Cellulases: From Lignocellulosic Biomass to Improved Production. Energies. 2023; 16(8): 3598. https://doi.org/10.3390/en16083598
[40] Miljkovic M, Davidovic S, Djukic-Vukovic A, et al. Utilization of agro-industrial by-products as substrates for dextransucrase production by Leuconostoc mesenteroides T3: Process optimization using response surface methodology. Hem Ind. 2021; 75(3): 135-146. https://doi.org/10.2298/HEMIND200710015M
[41] Mihajlovski K, Davidovic S, Veljovic D, Carevic M, Lazic V, Dimitrijevic-Brankovic S. Effective valorization of barley bran for simultaneous cellulase and β-amylase production by Paenibacillus chitinolyticus CKS1: Statistical optimization and enzymes application. J Serbian Chem Soc. 2017; 82(11): 1223-1236. https://doi.org/10.2298/JSC170514092M
[42] Salim AA, Grbavčić S, Šekuljica N, et al. Enzyme production by solid‐state fermentation on soybean meal: A comparative study of conventional and ultrasound‐assisted extraction methods. Biotechnol Appl Biochem. 2019; 66(3): 361-368. https://doi.org/10.1002/bab.1732
[43] Ademakinwa AN, Agboola FK. Kinetic and thermodynamic investigations of cell-wall degrading enzymes produced by Aureobasidium pullulans via induction with orange peels: application in lycopene extraction. Prep Biochem Biotechnol. 2019; 49(10): 949-60. https://doi.org/10.1080/10826068.2019.1650375
[44] Gostinčar C, Ohm RA, Kogej T, et al. Genome sequencing of four Aureobasidium pullulans varieties: Biotechnological potential, stress tolerance, and description of new species. BMC Genomics. 2014;.15(1): 549 . https://doi.org/10.1186/1471-2164-15-549
[45] Yegin S, Buyukkileci AO, Sargin S, Goksungur Y. Exploitation of Agricultural Wastes and By-Products for Production of Aureobasidium pullulans Y-2311-1 Xylanase: Screening, Bioprocess Optimization and Scale Up. Waste and Biomass Valorization. 2017; 8(3): 999-1010. https://doi.org/10.1007/s12649-016-9646-6
[46] Bennamoun L, Hiligsmann S, Dakhmouche S, et al. Production and Properties of a Thermostable, pH—Stable Exo-Polygalacturonase Using Aureobasidium pullulans Isolated from Saharan Soil of Algeria Grown on Tomato Pomace. Foods. 2016; 5(4): 72. https://doi.org/10.3390/foods5040072
[47] Alhomodi AF, Gibbons WR, Karki B. Estimation of cellulase production by Aureobasidium pullulans, Neurospora crassa, and Trichoderma reesei during solid and submerged state fermentation of raw and processed canola meal. Bioresour Technol Reports. 2022; 18: 101063. https://doi.org/10.1016/j.biteb.2022.101063
[48] Vieira MM, Kadoguchi E, Segato F, da Silva SS, Chandel AK. Production of cellulases by Aureobasidium pullulans LB83: optimization, characterization, and hydrolytic potential for the production of cellulosic sugars. Prep Biochem Biotechnol 2021; 51(2): 153-163. https://doi.org/10.1080/10826068.2020.1799393
[49] Qian Y, Zhong L, Sun Y, et al. Enhancement of Cellulase Production in Trichoderma reesei via Disruption of Multiple Protease Genes Identified by Comparative Secretomics. Front Microbiol. 2019; 10. https://doi.org/10.3389/fmicb.2019.02784