Poly(methacrylic acid) hydrogels crosslinked by poly(ethylene glycol) diacrylate as pH-responsive systems for drug delivery applications Original scientific paper
Main Article Content
Abstract
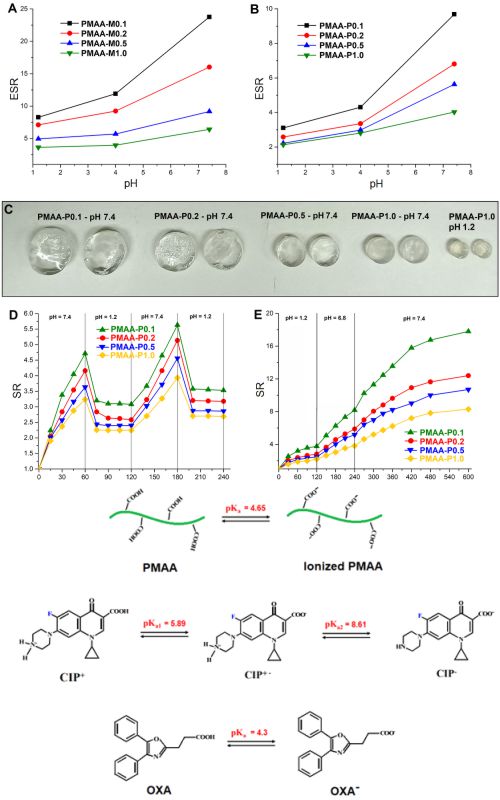
Hydrogels are attractive materials for drug delivery applications due to biocompatible, porous structure with the possibility to load and deliver drugs in a controllable manner. In this paper, poly(methacrylic acid) (PMAA) hydrogels are described, which are synthesized by free-radical polymerization, using poly(ethylene glycol) diacrylate (PEGDA) as a crosslinker. Influence of the PEGDA content on hydrogel properties was investigated and compared to commonly used crosslinker - N,N’-methylenebisacrylamide (MBA). The increasing concentration of crosslinkers led to a higher degree of crosslinking, which was demonstrated by a higher degree of conversion, lower swelling capacity, and improved thermal stability and mechanical properties. Also, the PEGDA-crosslinked hydrogels demonstrated a higher degree of crosslinking than the corresponding MBA-crosslinked hydrogels. Potential application of the synthesized hydrogels for controlled drug delivery was investigated by using two model drugs - oxaprozin and ciprofloxacin. In vitro drug release tests indicated that the interactions between drug, polymer and medium have a key influence on the drug release behavior, rather than the swelling rate. Drug release tests in simulated gastrointestinal conditions indicated that PEGDA-crosslinked PMAA hydrogels are suitable for colon-targeted delivery of oxaprozin.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-68/2022-14/200287;451-03-68/2022-14/200135;451-03-9/2021-14/200026;451-03-68/2022-14/200178
References
Bashari A, Rouhani Shirvan A, Shakeri M. Cellulose-based hydrogels for personal care products. Polym Adv Technol. 2018; 29(12): 2853-2867 https://doi.org/10.1002/pat.4290.
Valuev LI, Valuev IL, Vanchugova L V, Obydennova I V. Glucose-Sensitive Hydrogels for the Controlled Release of Insulin. Polym Sci Ser A. 2018; 60(4): 495-498 https://doi.org/10.1134/S0965545X18040132.
Popova E V, Morozova P V, Uspenskaya M V, Radilov AS. Sodium alginate and carbopol microcapsules: preparation, polyphenol encapsulation and release efficiency. Russ Chem Bull. 2021; 70(7): 1335-1340 https://doi.org/10.1007/s11172-021-3220-5.
Bogdanova LR, Rogov AM, Zueva OS, Zuev YF. Lipase enzymatic microreactor in polysaccharide hydrogel: structure and properties. Russ Chem Bull. 2019; 68(2): 400-404 https://doi.org/10.1007/s11172-019-2399-1.
Kadimaliev DA, Devyataeva AA, Grunyushkin IP, Malafeev AN, Revin V V. Influence of Bacterial Cellulose Gel Film Modification on Its Mechanical Properties and Ability to Covalently Bind Enzymes. Polym Sci Ser B. 2021; 63(3): 232-238 https://doi.org/10.1134/S1560090421030088.
Shewan HM, Stokes JR. Review of techniques to manufacture micro-hydrogel particles for the food industry and their applications. J Food Eng. 2013; 119(4): 781-792 https://doi.org/10.1016/j.jfoodeng.2013.06.046.
Ilyasov LO, Panova IG, Khrabrov NA, Kushchev PO, Loiko NG, Nikolaev YA, Yaroslavov AA. Loosely Crosslinked Hydrogel with Combined Water-Retaining and Anti-Erosion Effect. Polym Sci Ser B. 2021; 63(6): 866-873 https://doi.org/10.1134/S1560090421060105.
Panova IG, Ilyasov LO, Khaidapova DD, Ogawa K, Adachi Y, Yaroslavov AA. Polyelectrolytic Gels for Stabilizing Sand Soil against Wind Erosion. Polym Sci Ser B. 2020; 62(5): 491-498 https://doi.org/10.1134/S1560090420050103.
Temel S, Yaman E, Ozbay N, Gokmen FO. Synthesis, characterization and adsorption studies of nano-composite hydrogels and the effect of SiO2 on the capacity for the removal of Methylene Blue dye. J Serb Chem Soc. 2020; 85(7): 939-952 https://doi.org/10.2298/JSC190517114T.
Bogdanova LR, Makarova AO, Zueva OS, Zakharova LY, Zuev YF. Encapsulation of diagnostic dyes in the polysaccharide matrix modified by carbon nanotubes. Russ Chem Bull. 2020; 69(3): 590-595 https://doi.org/10.1007/s11172-020-2803-x.
Huang X, Wang C, Ao X, Li C, Yang L. Preparation and Properties of Cellulose Nanofiber-Reinforced Ionic Conductive Hydrogels Sensor. Polym Sci Ser A. 2022; 64(6): 765-774 https://doi.org/10.1134/S0965545X22700420.
Radonjić M, Petrović J, Milivojević M, Stevanović M, Stojkovska J, Obradović B. Chemical engineering methods in analyses of 3d cancer cell cultures: hydrodynamic and mass transport considerations: Scientific paper. Chem Ind Chem Eng Q. 2022; 28(3): 211-223 https://doi.org/10.2298/CICEQ210607033R.
Sultanova EM, Oripova MZ, Oshchepkova YI, Salikhov SI. Chitosan-Based Hydrogel Composition with Megosine. Pharm Chem J. 2020; 54(5): 514-517 https://doi.org/10.1007/s11094-020-02230-x.
Gorshkova MY, Vanchugova L V, Volkova IF, Obydennova I V, Valuev IL, Valuev LI. Novel mucoadhesive carriers based on alginate-acrylamide hydrogels for drug delivery. Mendeleev Commun. 2022; 32(2): 189-191 https://doi.org/10.1016/j.mencom.2022.03.012.
Len’shina NA, Konev AN, Baten’kin AA, et al. Alginate Functionalization for the Microencapsulation of Insulin Producing Cells. Polym Sci Ser B. 2021; 63(6): 640-656 https://doi.org/10.1134/S1560090421060129.
Dubashynskaya N V, Petrova VA, Romanov DP, Skorik YA. pH-Sensitive Drug Delivery System Based on Chitin Nanowhiskers-Sodium Alginate Polyelectrolyte Complex. Materials. 2022; 15(17): 5860 https://doi.org/10.3390/ma15175860.
Gorshkova MY, Volkova IF, Grigoryan ES, Valuev LI. Sodium Alginate Interpolymer Complexes as a Platform for pH-Tunable Drug Carriers. Polym Sci Ser B. 2020; 62(6): 678-684 https://doi.org/10.1134/S1560090420060044.
Odinokov A V, Dzhons DY, Budruev A V, Mochalova AE, Smirnova LA. Chitosan modified with terephthaloyl diazide as a drug delivery system. Russ Chem Bull. 2016; 65(4): 1122-1130 https://doi.org/10.1007/s11172-016-1423-y.
Mirković I, Nikolić MS, Ostojić S, Maletaškić J, Petrović Z, Djonlagić J. Thermo-responsive hydrogels based on poly(N-isopropyl-acrylamide) and hyaluronic acid cross-linked with nanoclays. J Serb Chem Soc. 2020; 85(9): 1197-1221 https://doi.org/10.2298/JSC200109023M.
Urošević MZ, Nikolić LB, Ilić-Stojanović S, Zdravković A, Nikolić V. Synthesis and characterization of poly(N-isopropylmethacrylamide-co-N-isopropylacrylamide) copolymers. Hem Ind. 2020; 74(2): 103-117 https://doi.org/10.2298/HEMIND190717007U.
Xiang T, Lu T, Zhao W-F, Zhao C-S. Ionic-Strength Responsive Zwitterionic Copolymer Hydrogels with Tunable Swelling and Adsorption Behaviors. Langmuir. 2019; 35(5): 1146-1155 https://doi.org/10.1021/acs.langmuir.8b01719.
Zhao Y-L, Stoddart JF. Azobenzene-Based Light-Responsive Hydrogel System. Langmuir. 2009; 25(15): 8442-8446 https://doi.org/10.1021/la804316u.
Markovic MD, Seslija SI, Ugrinovic VD, Kunaver M, Panic VV, Pjanovic RV, Spasojevic PM. Green pH- and magnetic-responsive hybrid hydrogels based on poly(methacrylic acid) and Eucalyptus wood nanocellulose for controlled release of ibuprofen. Cellulose. 2021; 28(17): 11109-11132 https://doi.org/10.1007/s10570-021-04222-w.
Qi X, Wei W, Li J, Liu Y, Hu X, Zhang J, Bi L, Dong W. Fabrication and Characterization of a Novel Anticancer Drug Delivery System: Salecan/Poly(methacrylic acid) Semi-interpenetrating Polymer Network Hydrogel. ACS Biomater Sci Eng. 2015; 1(12): 1287-1299 https://doi.org/10.1021/acsbiomaterials.5b00346.
Panic V, Adnadjevic B, Velickovic S, Jovanovic J. The effects of the synthesis parameters on the xerogels structures and on the swelling parameters of the poly(methacrylic acid) hydrogels. Chem Eng J. 2010; 156(1): 206-214 https://doi.org/10.1016/J.CEJ.2009.10.040.
Prusty K, Biswal A, Biswal SB, Swain SK. Synthesis of soy protein/polyacrylamide nanocomposite hydrogels for delivery of ciprofloxacin drug. Mater Chem Phys. 2019; 234: 378-389 https://doi.org/10.1016/j.matchemphys.2019.05.038.
Markovic MD, Panic VV, Seslija SI, Spasojevic PM, Ugrinovic VD, Boskovic-Vragolovic NM, Pjanovic RV. Modification of hydrophilic polymer network to design a carrier for a poorly water-soluble substance. Polym Eng Sci. 2020; 60(10): 2496-2510 https://doi.org/10.1002/pen.25487.
Ugrinovic V, Panic V, Spasojevic P, Seslija S, Bozic B, Petrovic R, Janackovic D, Veljovic D. Strong and tough, pH sensible, interpenetrating network hydrogels based on gelatin and poly(methacrylic acid). Polym Eng Sci. 2022; 62(3): 622-636 https://doi.org/10.1002/pen.25870.
Das D, Ghosh P, Dhara S, Panda AB, Pal S. Dextrin and Poly(acrylic acid)-Based Biodegradable, Non-Cytotoxic, Chemically Cross-Linked Hydrogel for Sustained Release of Ornidazole and Ciprofloxacin. ACS Appl Mater Interfaces. 2015; 7(8): 4791-4803 https://doi.org/10.1021/am508712e.
Fan W, Zhang Z, Liu Y, Wang J, Li Z, Wang M. Shape memory polyacrylamide/gelatin hydrogel with controllable mechanical and drug release properties potential for wound dressing application. Polymer. 2021; 226: 123786 https://doi.org/10.1016/j.polymer.2021.123786.
Tu C-W, Tsai F-C, Chen J-K, et al. Preparations of Tough and Conductive PAMPS/PAA Double Network Hydrogels Containing Cellulose Nanofibers and Polypyrroles. Polymers. 2020; 12(12): 2835 https://doi.org/10.3390/polym12122835.
D’souza AA, Shegokar R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv. 2016; 13(9): 1257-1275 https://doi.org/10.1080/17425247.2016.1182485.
Koga T, Tomimori K, Higashi N. Transparent, High-Strength, and Shape Memory Hydrogels from Thermo-Responsive Amino Acid-Derived Vinyl Polymer Networks. Macromol Rapid Commun. 2020; 41(7): 1900650 https://doi.org/10.1002/marc.201900650.
Nalampang K, Panjakha R, Molloy R, Tighe BJ. Structural effects in photopolymerized sodium AMPS hydrogels crosslinked with poly(ethylene glycol) diacrylate for use as burn dressings. J Biomater Sci Polym Ed. 2013; 24(11): 1291-1304 https://doi.org/10.1080/09205063.2012.755601.
Zhong C, Wu J, Reinhart-King CA, Chu CC. Synthesis, characterization and cytotoxicity of photo-crosslinked maleic chitosan-polyethylene glycol diacrylate hybrid hydrogels. Acta Biomater 2010; 6(10): 3908-3918 https://doi.org/10.1016/j.actbio.2010.04.011.
Cao H, Wang Q, Li M, Chen Z. Synthesis of stimuli-responsive poly(ethylene glycol) diacrylate/methacrylic acid-based nanogels and their application as drug delivery vehicle. Colloid Polym Sci. 2015; 293(2): 441-451 https://doi.org/10.1007/s00396-014-3422-6.
Das D, Pal S. Dextrin/poly (HEMA): pH responsive porous hydrogel for controlled release of ciprofloxacin. Int J Biol Macromol. 2015; 72: 171-178 https://doi.org/10.1016/j.ijbiomac.2014.08.007.
Hanna DH, Saad GR. Encapsulation of ciprofloxacin within modified xanthan gum- chitosan based hydrogel for drug delivery. Bioorg Chem. 2019; 84: 115-124 https://doi.org/10.1016/j.bioorg.2018.11.036.
Lazou M, Hatzidimitriou AG, Papadopoulos AN, Psomas G. Zinc-oxaprozin compounds: Synthesis, structure and biological activity. J Inorg Biochem. 2019; 195: 101-110 https://doi.org/10.1016/j.jinorgbio.2019.03.016.
Jamar R, Dequeker J. Oxaprozin versus aspirin in rheumatoid arthritis: a double-blind trial. Curr Med Res Opin. 1978; 5(6): 433-438 https://doi.org/10.1185/03007997809111911.
Peng S, Duggan A. Gastrointestinal adverse effects of non-steroidal anti-inflammatory drugs. Expert Opin Drug Saf. 2005; 4(2): 157-169 https://doi.org/10.1517/14740338.4.2.157.
Brown K. Oxazoles. U.S. Patent No. 3,578,671, 1971
Božić B, Rogan J, Poleti D, Trišović N, Božić B, Ušćumlić G. Synthesis, characterization and antiproliferative activity of transition metal complexes with 3-(4, 5-diphenyl-1, 3-oxazol-2-yl) propanoic acid (oxaprozin). Chem Pharm Bull. 2012; 60(7): 865-869 https://doi.org/10.1248/cpb.c12-00185.
Ribeiro LNM, Alcântara ACS, Darder M, Aranda P, Araújo-Moreira FM, Ruiz-Hitzky E. Pectin-coated chitosan-LDH bionanocomposite beads as potential systems for colon-targeted drug delivery. Int J Pharm. 2014; 463(1): 1-9 https://doi.org/10.1016/j.ijpharm.2013.12.035.
Hervás Pérez JP, López-Ruiz B, López-Cabarcos E. Synthesis and characterization of microparticles based on poly-methacrylic acid with glucose oxidase for biosensor applications. Talanta. 2016; 149: 310-318 https://doi.org/10.1016/j.talanta.2015.11.053.
McAvoy K, Jones D, Thakur RRS. Synthesis and Characterisation of Photocrosslinked poly(ethylene glycol) diacrylate Implants for Sustained Ocular Drug Delivery. Pharm Res. 2018; 35(2): 36 https://doi.org/10.1007/s11095-017-2298-9.
Lei J, Mayer C, Freger V, Ulbricht M. Synthesis and Characterization of Poly(ethylene glycol) Methacrylate Based Hydrogel Networks for Anti-Biofouling Applications. Macromol Mater Eng. 2013; 298(9): 967-980 https://doi.org/10.1002/mame.201200297.
Kucharski M, Lubczak R. Copolymerization of hydroxyalkyl methacrylates with acrylamide and methacrylamide I. Determination of reactivity ratios. J Appl Polym Sci. 1997; 64(7): 1259-1265 https://doi.org/10.1002/(SICI)1097-4628(19970516)64:7<1259::AID-APP3>3.0.CO;2-I.
Fyfe CA, McKinnon MS. Investigation of the thermal degradation of poly(acrylic acid) and poly(methacrylic acid) by high-resolution carbon-13 CP/MAS NMR spectroscopy. Macromolecules. 1986; 19(7): 1909-1912 https://doi.org/10.1021/ma00161a021.
Schild HG. Thermal degradation of poly(methacrylic acid): Further studies applying TGA/FTIR. J Polym Sci Part A Polym Chem. 1993; 31(9): 2403-2405 https://doi.org/10.1002/pola.1993.080310925.
Yang F, Zhao J, Koshut WJ, Watt J, Riboh JC, Gall K, Wiley BJ. A Synthetic Hydrogel Composite with the Mechanical Behavior and Durability of Cartilage. Adv Funct Mater. 2020; 30(36): 2003451 https://doi.org/10.1002/adfm.202003451.
Czyrski A. The spectrophotometric determination of lipophilicity and dissociation constants of ciprofloxacin and levofloxacin. Spectrochim Acta Part A Mol Biomol Spectrosc. 2022; 265: 120343 https://doi.org/10.1016/j.saa.2021.120343.