Application of impregnated biocarbon produced from soybean hulls in dye decolorization
Main Article Content
Abstract
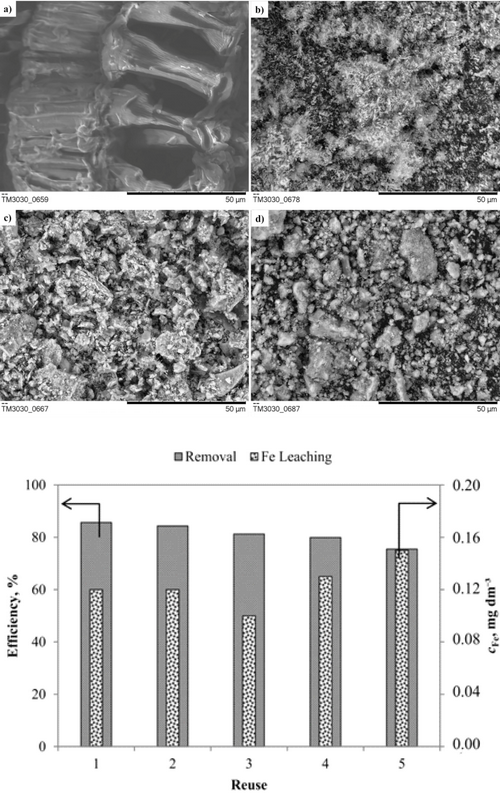
Waste soybean hulls (WSH) were investigated as a Fe-support in two forms: raw and carbonized (i.e. biocarbon, BC), as possible value-added materials. Fe-impregnation was implemented in order to produce heterogeneous Fenton catalysts for Reactive Blue 4 dye degradation. Materials characterization demonstrated a rise in the specific surface area due to decomposition of WSH constituents during carbonization (to obtain BC) and thermal activation (to obtain Fe-WSH and Fe-BC), thus producing catalysts with high mesoporosity and hematite as the active site for Fenton reaction. Among the investigated materials, Fe‑WSH showed the greatest ability for •OH production in acidic medium. Next, the heterogeneous Fenton process was optimized by using response surface methodology, which resulted in selection of the following reaction conditions: 3 mM H2O2, 100 mg Fe-WSH, reaction time of 180 min, at a constant pH 3, RB4 concentration of 50 mg dm-3 and at room temperature. The achieved dye removal and mineralization were 85.7 and 66.8 %, respectively, while the catalyst showed high stability and the reaction intermediates formed during the oxidation process had a low inhibitory effect on Vibrio fischeri bacteria.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
References
Holkar CR, Jadhav AJ, Pinjari DV, Mahamuni NM, Pandit AB. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manage. 2016; 182: 351–366. https://dx.doi.org/10.1016/j.jenvman.2016.07.090
Gözmen B, Kayan B, Gizir M, Heresnov A. Oxidative degradations of reactive blue 4 dye by different advanced oxidation methods. J. Hazard. Mater. 2009; 168: 129–136. https://dx.doi.org/10.1016/j.jhazmat.2009.02.011
Hassan H, Hameed BH. Fe-Natural Zeolite as Highly Active Heterogeneous Catalyst in Decolorization of Reactive Blue 4. Int. J. Environ. Sci. Dev. 2020; 11: 133–137. https://dx.doi.org/10.18178/ijesd.2020.11.3.1239
Fatma NY, Riyanti F, Hariani PL, Nurbaiti B. Synthesis of chitosan/alumina composite by sol gel method for adsorption of procion blue MX-R dye from wastewater songket industry. J. Phys. Conf. Ser. 2019; 1282: 012080. https://doi.org/10.1088/1742-6596/1282/1/012080
da Silva RG, de Andrade AR. Degradation of the Dye Reactive Blue 4 by Coupled Photoassisted Electrochemistry at DSA®-Type Electrode. J. Brazil. Chem. Soc. 2016; 27: 857–865. http://dx.doi.org/10.5935/0103-5053.20150338
Tomin MB, Kulic A, Kerkez D, Prica M, Rapajic S, Pilipovic DT, Pesic V. Reactive dye degradation using Fe-loaded bentonite as a Fenton-like catalyst: From process optimization to effluent acute toxicity. Fresen. Environ. Bull. 2017; 26: 8184–8198. https://www.prt-parlar.de/download_feb_2017/
Zhang M, Dong H, Zhao L, Wang D, Meng D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019; 670: 110–121. https://dx.doi.org/10.1016/j.scitotenv.2019.03.180
Eloussaief M, Hamza W, Ghorbali G, Kallel N, Benzina M. Fe-Rich Aragonite Concretion Applied to Industrial Dye Purification Using Fenton and Photo-Fenton Technologies. Waste Biomass Valori. 2021; 12: 3303-3313. https://dx.doi.org/10.1007/s12649-020-01228-6
Wang N, Zheng T, Zhang G, Wang P. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016; 4: 762–787. https://dx.doi.org/10.1016/j.jece.2015.12.016
Javaid R, Qazi UY. Catalytic oxidation process for the degradation of synthetic dyes: An overview. Int. J. Environ. Res. Public Health. 2019; 16: 2066. https://dx.doi.org/10.3390/ijerph16112066
Zhu Y, Zhu R, Xi Y, Zhu J, Zhu G, He H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B Environ. 2019; 255: 117739. https://doi.org/10.1016/j.apcatb.2019.05.041
Bello M, Raman AA, Asghar A. A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Process Saf. Environ. Prot. 2019; 126: 119-140. https://doi.org/10.1016/j.psep.2019.03.028
Nidheesh PV. Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: A review. RSC Adv. 2015; 5: 40552–40577. https://dx.doi.org/10.1039/c5ra02023a
Wang J, Bai Z. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater. Chem. Eng. J. 2017; 312: 79–98. https://dx.doi.org/10.1016/j.cej.2016.11.118
Girish CR. Various impregnation methods used for the surface modification of the adsorbent: A review. Int. J. Eng. Technol. 2018; 7: 330–334. https://dx.doi.org/10.14419/ijet.v7i4.7.20571
Elías VR, Rodriguez PAO, Vaschetto EG, Pecchi GA, Huck-Iriart C, Casuscelli SG, Eimer GA. Tailoring the stability and photo-Fenton activity of Fe-modified nanostructured silicates by tuning the metal speciation from different synthesis conditions. Mol. Catal. 2020; 481: 110217. https://dx.doi.org/10.1016/j.mcat.2018.10.012
Setiawan WK, Chiang KY. Crop Residues as Potential Sustainable Precursors for Developing Silica Materials: A Review. Waste Biomass Valori. 2020; 2207-2236. https://dx.doi.org/10.1007/s12649-020-01126-x
Campbell-Johnston K, Vermeulen WJV, Reike D, Brullot S. The Circular Economy and Cascading: Towards a Framework. Resour. Conserv. Recycl. X. 2020; 7: 100038. https://dx.doi.org/10.1016/j.rcrx.2020.100038
Xiang W, Zhang X, Chen J, Zou W, He F, Hu X, Tsang DCW, Ok YS, Gao B. Biochar technology in wastewater treatment: A critical review. Chemosphere. 2020; 252: 126539. https://dx.doi.org/10.1016/j.chemosphere.2020.126539
Enaime G, Baçaoui A, Yaacoubi A, Lübken M. Biochar for wastewater treatment-conversion technologies and applications. Appl. Sci. 2020; 10: 3492. https://dx.doi.org/10.3390/app10103492
Pan X, Gu Z, Chen W, Li Q. Preparation of biochar and biochar composites and their application in a Fenton-like process for wastewater decontamination: A review. Sci. Total Environ. 2021; 754: 142104. https://dx.doi.org/10.1016/j.scitotenv.2020.142104
Statistical Office of the Republic of Serbia. Statistical Release PO16: Realized production of wheat and early fruit and expected yields of late crops, fruit and grapes, 2020. Agric. Stat. 2020; 262: 1-2. ISSN 0353-9555
Barros PJR, Ascheri DPR, Santos MLS, Morais CC, Ascheri JLR, Signini R, dos Santos DM, de Campos AJ, Devilla IA. Soybean hulls: Optimization of the pulping and bleaching processes and carboxymethyl cellulose synthesis. Int. J. Biol. Macromol. 2020; 144: 208–218. https://dx.doi.org/10.1016/j.ijbiomac.2019.12.074
Liu H-M, Li H-Y. Application and Conversion of Soybean Hulls. In: Kasai M, ed. Soybean - The Basis of Yield, Biomass and Productivity. IntechOpen; 2017: 111–132. https://dx.doi.org/10.5772/66744
Qing Q, Guo Q, Zhou L, Gao X, Lu X, Zhang Y. Comparison of alkaline and acid pretreatments for enzymatic hydrolysis of soybean hull and soybean straw to produce fermentable sugars. Ind. Crops Prod. 2017; 109: 391–397. https://dx.doi.org/10.1016/j.indcrop.2017.08.051
Neto WPF, Silvério HA, Dantas NO, Pasquini D. Extraction and characterization of cellulose nanocrystals from agro-industrial residue - Soy hulls. Ind. Crops Prod. 2013; 42: 480–488. https://dx.doi.org/10.1016/j.indcrop.2012.06.041
Toro-Trochez JL, Carrillo-Pedraza ES, Bustos-Martínez D, García-Mateos FJ, Ruiz-Rosas RR, Rodríguez-Mirasol J, Cordero T. Thermogravimetric characterization and pyrolysis of soybean hulls. Bioresour. Technol. Reports. 2019; 6: 183–189. https://dx.doi.org/10.1016/j.biteb.2019.02.009
Herde ZD, Dharmasena R, Sumanasekera G, Tumuluru JS, Satyavolu J. Impact of hydrolysis on surface area and energy storage applications of activated carbons produced from corn fiber and soy hulls. Carbon Resour. Convers. 2020; 3: 19–28. https://dx.doi.org/10.1016/j.crcon.2019.12.002
Bečelić-Tomin M, Kulić A, Kerkez Đ, Pilipović DT, Pešić V, Dalmacija B. Synthesis of impregnated bentonite using ultrasound waves for application in the Fenton process. Clay Miner. 2018; 53: 203–212. https://dx.doi.org/10.1180/clm.2018.14
Xiao C, Li J, Zhang G. Synthesis of stable burger-like α-Fe2O3 catalysts: Formation mechanism and excellent photo-Fenton catalytic performance. J. Clean. Prod. 2018; 180: 550–559. https://dx.doi.org/10.1016/j.jclepro.2018.01.127
Trovó AG, Nogueira RFP, Agüera A, Fernandez-Alba AR, Malato S. Degradation of the antibiotic amoxicillin by photo-Fenton process - Chemical and toxicological assessment. Water Res. 2011; 45: 1394–1402. https://dx.doi.org/10.1016/j.watres.2010.10.029
Milidrag GP, Prica M, Kerkez D, Dalmacija B, Kulic A, Pilipovic DT, Tomin MB. A comparative study of the decolorization capacity of the solar-assisted Fenton process using ferrioxalate and Al, Fe-bentonite catalysts in a parabolic trough reactor. J. Taiwan Inst. Chem. Eng. 2018; 93: 436–449. https://dx.doi.org/10.1016/j.jtice.2018.08.015
Balint T, Chang BP, Mohanty AK, Misra M. Underutilized Agricultural Co-Product as a Sustainable Biofiller for Polyamide 6,6: Effect of Carbonization Temperature. Molecules. 2020; 25: 1455. https://dx.doi.org/doi:10.3390/molecules25061455
Wu Q, Ouyang J, Xie K, Sun L, Wang M, Lin C. Ultrasound-assisted synthesis and visible-light-driven photocatalytic activity of Fe-incorporated TiO2 nanotube array photocatalysts. J. Hazard. Mater. 2012; 199–200: 410–417. https://dx.doi.org/10.1016/j.jhazmat.2011.11.031
Nandiyanto ABD, Oktiani R, Ragadhita R. How to read and interpret ftir spectroscope of organic material. Indones. J. Sci. Technol. 2019; 4: 97–118. https://dx.doi.org/10.17509/ijost.v4i1.15806
Chen Y, Shi J, Du Q, Zhang H, Cui Y. Antibiotic removal by agricultural waste biochars with different forms of iron oxide. RSC Adv. 2019; 9: 14143–14153. https://dx.doi.org/10.1039/c9ra01271k
Wang QJ, Liu RJ, Shen XQ, Wu DM, Li HH. Fabrication and methyl blue adsorption kinetics of α-Fe2O3 nanotubes by electrospinning. Adv. Mater. Res. 2013; 699: 302–307. https://dx.doi.org/10.4028/www.scientific.net/AMR.699.302
Galan J, Trilleras J, Zapata PA, Arana VA, Grande-Tovar CD. Optimization of chitosan glutaraldehyde-cross linked beads for reactive blue 4 anionic dye removal using a surface response methodology. Life. 2021; 11: 1–20. https://dx.doi.org/10.3390/life11020085
Zhao L, Lin ZR, Ma XH, Dong, YH. Catalytic activity of different iron oxides: Insight from pollutant degradation and hydroxyl radical formation in heterogeneous Fenton-like systems. Chem. Eng. J. 2018; 352: 343–351. https://dx.doi.org/10.1016/j.cej.2018.07.035
Becelic-Tomin M, Dalmacija B, Rajic L, Tomasevic D, Kerkez D, Watson M, Prica M. Degradation of anthraquinone dye reactive blue 4 in pyrite ash catalyzed fenton reaction. Sci. World J. 2014; 234654. https://dx.doi.org/10.1155/2014/234654
Schneider JT, Firak DS, Ribeiro RR, Peralta-Zamora P. Use of scavenger agents in heterogeneous photocatalysis: truths, half-truths, and misinterpretations. Phys. Chem. Chem. Phys. 2020; 22: 15723–15733. https://dx.doi.org/10.1039/d0cp02411b
Stupar SL, Grgur BN, Radišić MM, Onjia AE, Ivanković ND, Tomašević AV, Mijin D. Oxidative degradation of Acid Blue 111 by electro-assisted Fenton process. J. Water Process Eng. 2020; 36: 101394. https://dx.doi.org/10.1016/j.jwpe.2020.101394