Kinetika faznih transformacija u sintezi barijum-titanata mehanohemijskom obradom Naučni rad
Glavni sadržaj članka
Apstrakt
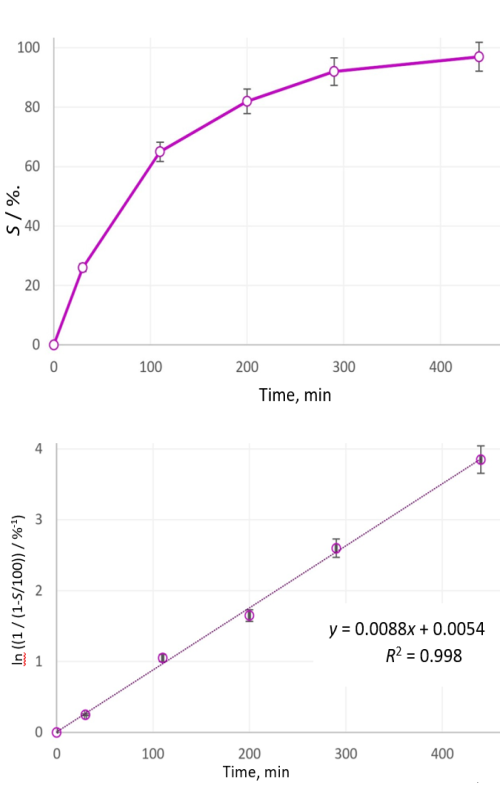
U radu su prikazani rezultati istraživanja sinteze barijum titanata (BaTiO3) na niskim temperaturama suvim mehanohemijskim postupkom. Bazni reaktant u eksperimentima je barijum oksid (BaO), dok je kao kiseli reaktant bio titanijum dioksid (TiO2). Optimalna količina polaznih uzoraka za aktivaciju je bila od 50 do 150 g. U cilju praćenja reakcije između polaznih uzoraka, reakciona smeša je uzorkovana nakon 30, 110, 200, 290 i 440 min aktivacije. Za reakcije neutralizacije između BaO i TiO2 korišćen je visokoenergetski vibracioni mlin sa torzionim oprugama i prstenastim radnim elementima. Produkti mehanohemijske reakcije su hemijski analizirani u cilju identifikacije neizreagovanih ostataka oksida zemnoalkalnih metala čija količina može da ukaže na stepen konverzije ili sinteze. Za identifikaciju kristalnih formi nastalih tokom reakcije i praćenje faznih transformacija korišćena je difrakciona rendgenska analiza praha (engl. X-ray powder diffraction, XRD). Dobijeni rezultati ovom analizom su omogućili definisanje dinamike sinteze. Sastav reakcione smeše, u tačno definisanim vremenskim intervalima tokom postupka sinteze, kvantitativno je analiziran atomskom apsorpcionom spektroskopijom. Cilj ovog istraživanja je bio da se tokom sinteze BaTiO3 odredi reakcioni model kao i kinetika reakcije. Rezultati dobijeni u prikazanom eksperimentu su pokazali da je tokom reakcije sinteze BaTiO3 u čvrstom stanju postignut izuzetno visok stepen konverzije (0,99). Uzorkovanjem reakcione smeše u pet različitih vremenskih intervala potvrđeno je prisustvo početnih prahova BaO i TiO2, zatim intermedijarnih jedinjenja i na kraju konačnog proizvoda, kristalnog BaTiO3. Analizom nastanka kristalnih struktura i njihove identifikacije ceo proces dobijanja BaTiO3 može se podeliti u tri etape: prva etapa u kojoj se urušava kristalna struktura reakcionog sistema BaO-TiO2 (do 30 min); druga etapa je nastanak prelaznog stanja gde je teško utvrditi kristalnu i hemijsku strukturu, dovedena mehanička energija sistemu se akumulira u materijalu što ima za posledicu povećanje potencijalne energije i hemijske reaktivnosti (do 110 min); treća etapa u kojoj dolazi do značajnog formiranja kristalnog BaTiO3 (posle 200 min). Rezultati su pokazali da je za dati sistem potrebno 440 min mehaničke aktivacije da se izvrši potpuna reakcija neutralizacije.
Detalji članka
Broj časopisa
Rubrika

Ovaj rad je pod Creative Commons Autorstvo 4.0 Internacionalna licenca.
Kada je rukopis prihvaćen za objavlјivanje, autori prenose autorska prava na izdavača. U slučaju da rukopis ne bude prihvaćen za štampu u časopisu, autori zadržavaju sva prava.
Na izdavača se prenose sledeća prava na rukopis, uklјučujući i dodatne materijale, i sve delove, izvode ili elemente rukopisa:
- pravo da reprodukuje i distribuira rukopis u štampanom obliku, uklјučujući i štampanje na zahtev;
- pravo na štampanje probnih primeraka, reprint i specijalnih izdanja rukopisa;
- pravo da rukopis prevede na druge jezike;
- pravo da rukopis reprodukuje koristeći fotomehanička ili slična sredstva, uklјučujući, ali ne ograničavajući se na fotokopiranje, i pravo da distribuira ove kopije;
- pravo da rukopis reprodukuje i distribuira elektronski ili optički koristeći sve nosioce podataka ili medija za pohranjivanje, a naročito u mašinski čitlјivoj/digitalizovanoj formi na nosačima podataka kao što su hard disk, CD-ROM, DVD, Blu-ray Disc (BD), mini disk, trake sa podacima, i pravo da reprodukuje i distribuira rukopis sa tih prenosnika podataka;
- pravo da sačuva rukopis u bazama podataka, uklјučujući i onlajn baze podataka, kao i pravo prenosa rukopisa u svim tehničkim sistemima i režimima;
- pravo da rukopis učini dostupnim javnosti ili zatvorenim grupama korisnika na osnovu pojedinačnih zahteva za upotrebu na monitoru ili drugim čitačima (uklјučujući i čitače elektonskih knjiga), i u štampanoj formi za korisnike, bilo putem interneta, onlajn servisa, ili putem internih ili eksternih mreža.
Kako citirati
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-66/2024-03/200023;451-03-65/2024-03/200131;451-03-66/2024-03/200026
Reference
[1] Vijatović M, Bobić J, Stojanović B. History and Challenges of Barium Titanate: Part I. Sci Sinter. 2008; 10L: 155-165 https://doi.org/10.2298/SOS0802155V
[2] Prado L, Resende N, Silva R, Egues S, Salazar-Banda G. Influence of the synthesis method on the preparation of barium titanate nanoparticles. Chem Eng Process: Process Intensif. 2016; 103: 12-20 https://doi.org/10.1016/j.cep.2015.09.011
[3] Kubota K, Seo T, Koide K, Hasegawa Y, Ito H. Olefin-accelerated solid-state C-N cross-coupling reactions using mechanochemistry. Nat Commun. 2019; 10: 111 https://doi.org/10.1038/s41467-018-08017-9
[4] Zhang X, Zhou KS, Liu M, Deng CM, Deng CG,Deng ZQ. Adsorbability and spreadability of calcium-magnesium-alumino-silicate (CMAS) on Al-modified 7YSZ thermal barrier coating. Ceram Int. 2016; 42(16): 19349-19356 https://doi.org/10.1016/j.ceramint.2016.09.106
[5] Oleynikov NN, Tretyakov YD, Shumyantzev AV. Concerning the activation energy of solid state reactions. J Solid State Chem. 1974;11 (4):340-343 https://doi.org/10.1016/S0022-4596(74)80039-3
[6] Gomes W. Definition of Rate Constant and Activation Energy in Solid State Reactions. Nature. 1961;192: 865-866 https://doi.org/10.1038/192865a0
[7] Behrens M. Meso-and nano-structuring of industrial Cu/ZnO/(Al2O3) catalysts. J Catal.2009;267 (1):24-29 https://doi.org/10.1016/j.jcat.2009.07.009
[8] Criado JM. On the determination of the activation energy of solid-state reactions from the maximum reaction rate of isothermal runs. J Therm Anal. 1981; 21: 155-157 https://doi.org/10.1007/BF01913708
[9] Lewis GN. The atom and the molecule. J Am Chem Soc. 1916; 38 (4): 762-785 https://doi.org/10.1021/ja02261a002
[10] Avvakumov EG. Mekhanicheskiemetodyaktivaciikhimicheskihprocesov.2rd ed., Novosibirsk, Nauka; 1986 (in Russian)
[11] Boldyrev VV. Mechanochemistry and mechanical activation of solids. Solid State Ion. 1993; 63-65:537-543 https://doi.org/10.1016/0167-2738(93)90157-X
[12] Lazarević ZŽ, Bobić J, Romčević NŽ, Paunović N, Stojanović BD. Study of Barium Bismuth Titanate Prepared by Mechanochemical Synthesis. Sci Sinter.2009; 41: 329-335 https://doi.org/10.2298/SOS0903329L
[13] Obradović N, Filipović S, Pavlović V, Mitrić M, Marković S, Mitić V,Đorđević N, Ristić MM. Isothermal sintering of barium-zinc-titanate ceramics. Ceram Int. 2011; 37(1): 21-27 https://doi.org/10.1016/j.ceramint.2010.07.001
[14] Zdujić M, Poleti D, Jovalekić C, Karanović L. The evolution of structure induced by intensive milling in the system 2Bi2O3•3TiO2. J Non-Cryst Solids. 2006;352 (28-29): 3058-3068 https://doi.org/10.1016/j.jnoncrysol.2006.03.072
[15] Buscaglia V, Randall CA. Size and scaling effects in barium titanate. J Eur Ceram. 2020; 40: 3744-3758 https://doi.org/10.1016/j.jeurceramsoc.2020.01.021
[16] Reynolds GJ. Electrical Properties of Thin-Film Capacitors Fabricated Using High Temperature Sputtered Modified Barium titanate. Materials. 2012; 5: 644-660 https://doi.org/10.3390/ma5040644
[17] Wei X, Liu Y, Zhao D, Ge SS. 3D printing of piezoelectric barium tinatate with high density form milled powders. J Eur Ceram. 2020; 43 (8):3297-3306 https://doi.org/10.1016/j.jeurceramsoc.2020.06.021
[18] Brzozowski E, Castro MS. Synthesis of barium titanate improved by modications in the kinetics of the solid state reaction. J Eur Ceram. 2000;20: 2347-2351 https://doi.org/10.1016/S0955-2219(00)00148-5
[19] Kozawa T, Onda A, Yanagisawa K. Accelerated formation of barium titanate by solid-state reaction in water vapour atmosphere. J Eur Ceram. 2009; 29: 3259-3264 https://doi.org/10.1016/j.jeurceramsoc.2009.05.031
[20] Apaydin F, Parlak TT, Yıldız K. Low temperature formation of barium titanate in solid state reaction by mechanical activation of BaCO3 and TiO2. Mater Res Express. 2019; 6: 126330 https://doi.org/10.1088/2053-1591/ab6c0d
[21] Ashiri R. On the solid-state formation of BaTiO3 nanocrystals from mechanically activated BaCO3 and TiO2 powders: innovative mechanochemical processing, the mechanism involved, and phase and nanostructure evolutions. RSC Adv. 2016; 6: 17138 https://doi.org/10.1039/C5RA22942A
[22] Ziegmann A, Schubert DW. Influence of the particle size and the filing degree of barium titanate filled silicone elastomers used as potential dielectric elastomers on the mechanical properties and the crosslinking density. MaterToday Commun. 2018; 14: 90-98 https://doi.org/10.1016/j.mtcomm.2017.12.013
[23] Uttam R, Yadav N, Kumar S, Dhar R. Strengthening of columnar hexagonal phase of a room temperature discotic liquid crystalline material by using ferroelectric barium titanate nanoparticles. J Mol Liq. 2019; 294: 111609 https://doi.org/10.1016/j.molliq.2019.111609
[24] Gu L, Li T, Xu Y, Sun C, Yang Z, Zhu D, Chen D. Effects of the particle size of BaTiO3 fillers on fabrication and dielectric properties of BaTiO3/Polymer/Al films for capacitor energy-storage application. Materials. 2019;12: 439 https://doi.org/10.3390/ma12030439
[25] Binhayeeniyi N, Sukwisute P, Nawae S, Muensit N. Energy conversion capacity of barium zirconatetitanate. Materials. 2020; 13: 315 https://doi.org/10.3390/ma13020315
[26] Polley C, Distler T, Detsch R, Lund H, Springer A, Boccaccini AR, Seitz H. 3D Printing of Piezoelectric Barium Titanate-Hydroxyapatite Scaffolds with Interconnected Porosity for Bone Tissue Engineering. Materials.2020; 13: 1773 https://doi.org/10.3390/ma13071773
[27] Jelinek M, Vanek P, Tolde Z. Buixaderas E, Kocourek T, Studnicka V, Drahokoupil J, Petzelt J, Remsa J, Tyunina M. PLD preparet bioactive BaTiO3 films on TiNb implants. Mater Sci Eng. C. 2017; 70: 334-339 https://doi.org/10.1016/j.msec.2016.08.072
[28] Stojanović BD, Simoes AZ, Paiva-Santos CO, Jovalekić C, Mitic VV, Varela JA. Mechanochemical synthesis of barium titanate. J Eur Ceram. 2005; 25: 1985-1989 https://doi.org/10.1016/j.jeurceramsoc.2005.03.003
[29] Đorđević NG, Matijašević SD, Mihajlovic SR, Stojanović JN, Radulović AM, Savić LjB. The Effect of Particle Size on the Crystallization LiGe2(PO4)3 Phase from Glass. Sci Sinter. 2025; 57: 43-52 https://doi.org/10.2298/SOS231111064D
[30] Vidojkovic VM. Proučavanje mehanizma i kinetike mehanohemijske sinteze neorganskih soli kod reakcija neutralizacije, Doktorska disertacija, Univerzitet u Beogradu, 2001.
[31] Roine A. HSC Chemistry for Windows Chemical Reaction and Equilibrium Software with Extensive Database. Version 2.03, Outokumpu Research Oy, Pori, Finland 1994
[32] Ptaček P, Opravil T, Šoukal F. Introducing the Effective Mass of Activated Complex and the Discussion on the Wave Function of this Instanton. IntechOpen, London UK, 2018. https://doi.org/10.5772/intechopen.70734
[33] Đorđević N, Vlahović M, Mihajlović S. X-ray structural analysis of the BaO and TiO2 starting compounds and initial mechanochemical activation. Undeground Mining Engineering.2023; 42: 37-46 https://doi.org/10.5937/podrad2342037Q
[34] Botta PM, Aglietti EF, López JMP. Mechanochemical effects on the kinetics of zinc titanate formation. J Mater Sci. 2004; 39:5195-5199 https://doi.org/10.1023/B:JMSC.0000039209.48875.25
[35] Revellame ED, Fortela DL, Sharp W, Hernandez R, Zappi ME. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean Eng Technol. 2020; 1: 100032 https://doi.org/10.1016/j.clet.2020.100032
[36] Dondur V. Hemijska kinetika, Beograd, Fakultet za fizičku-hemiju; 1992 (in Serbian)