Sinteza, karakterizacija i elektrohemijske osobine kobaltom dopirane fosfat volframove heteropoli kiseline i njene bronze Naučni rad
Glavni sadržaj članka
Apstrakt
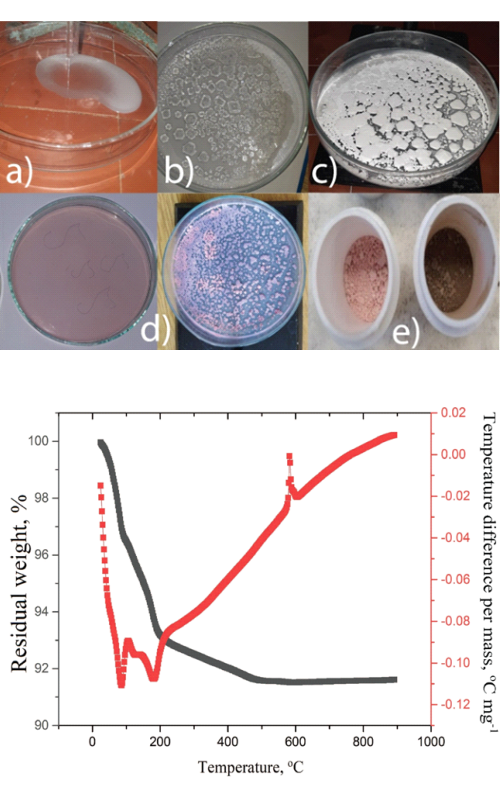
Heteropoli kiseline i njihova jedinjenja su fascinantna klasa multifunkcionalnih materijala koji se koriste u različitim naučnim oblastima: medicini, magnetizmu, katalizi, nelinearnoj optici kao i u elektrohemiji gde se primenjuju kao materijali za baterije. U ovoj studiji je polazna tačka volfram-fosfatna heteropoli kiselina iz koje je sintetisana i karakterisana njena kobaltova so (Co-PWA) i kobaltom dopirana volfram-fosfatna bronza (Co-PWB). Termička analiza je korišćena za određivanje faznog prelaza Co-PWA soli u Co-PWB bronzu koji se odvija na 588 °C. Ova temperatura je korišćena za žarenje Co-PWA da bi se sintetisala Co-PWB. Oba uzorka su dalje karakterisana korišćenjem infracrvene spektroskopije sa Furijeovom transformacijom (engl. Fourier transform infrared spectroscopy), difrakcije rendgenskih zraka na prahu (engl. X-ray powder diffraction) i skenirajuće elektronske mikroskopije koja sadrži energetsku disperzivnu rendgensku spektroskopiju (engl. scanning electron microscopy using an energy dispersive X-ray spectroscopy), kao i korišćenjem elektrohemijskih ispitivanja. Prisustvo kobalta je nedvosmisleno pokazano i u Co-PWA i Co-PWB, pri cemu je potvrdjeno uspešno dopiranje. Za elektrohemijska ispitivanja korišćene su ciklična voltametrija kao “brza” tehnika i metoda hronopotenciometrije radi simuliranja punjenja i pražnjenja baterije. Ciklična voltametrija je izmerila nestabilan i mali kapacitet za Co-PWA i stabilan za Co-PWB u vodenom rastvoru LiNO3. Razlog pada kapaciteta kod Co-PWA je nestabilnost ove soli u navedenom elektrolitu. Zbog stabilnog kapaciteta Co-PWB dobijenim merenjem cikličnom voltametrijom, ovaj material je podvrgnut hronopotenciometrijskom punjenju i pražnjenju pri strujama 1000, 2000 i 3000 mA g-1. Ovom metodom je pokazan stabilan kapacitet pri svakoj od primenjenih struja, što ga čini atraktivnim elektrodnim materijalom za vodene Li-jonske baterije. Dobijeni rezultati dopunjuju naučnu literaturu koja se bavi ispitivanjem sličnih materijala i doprinose boljem razumevanju karakteristika dopiranih kiselina i njihovih bronzi različitim metalima.
a.
Detalji članka
Broj časopisa
Rubrika

Ovaj rad je pod Creative Commons Autorstvo 4.0 Internacionalna licenca.
Kada je rukopis prihvaćen za objavlјivanje, autori prenose autorska prava na izdavača. U slučaju da rukopis ne bude prihvaćen za štampu u časopisu, autori zadržavaju sva prava.
Na izdavača se prenose sledeća prava na rukopis, uklјučujući i dodatne materijale, i sve delove, izvode ili elemente rukopisa:
- pravo da reprodukuje i distribuira rukopis u štampanom obliku, uklјučujući i štampanje na zahtev;
- pravo na štampanje probnih primeraka, reprint i specijalnih izdanja rukopisa;
- pravo da rukopis prevede na druge jezike;
- pravo da rukopis reprodukuje koristeći fotomehanička ili slična sredstva, uklјučujući, ali ne ograničavajući se na fotokopiranje, i pravo da distribuira ove kopije;
- pravo da rukopis reprodukuje i distribuira elektronski ili optički koristeći sve nosioce podataka ili medija za pohranjivanje, a naročito u mašinski čitlјivoj/digitalizovanoj formi na nosačima podataka kao što su hard disk, CD-ROM, DVD, Blu-ray Disc (BD), mini disk, trake sa podacima, i pravo da reprodukuje i distribuira rukopis sa tih prenosnika podataka;
- pravo da sačuva rukopis u bazama podataka, uklјučujući i onlajn baze podataka, kao i pravo prenosa rukopisa u svim tehničkim sistemima i režimima;
- pravo da rukopis učini dostupnim javnosti ili zatvorenim grupama korisnika na osnovu pojedinačnih zahteva za upotrebu na monitoru ili drugim čitačima (uklјučujući i čitače elektonskih knjiga), i u štampanoj formi za korisnike, bilo putem interneta, onlajn servisa, ili putem internih ili eksternih mreža.
Kako citirati
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-66/2024-03/200026;451-03-66/2024-03/200051;451-03-66/2024-03/200146;451-03-65/2024-03/200126 -
HORIZON EUROPE European Innovation Council
Grant numbers 101115149 -
Office of Naval Research Global
Grant numbers N62902-22-1-2024
Reference
[1] Kamiya Y, Okuhara T, Misono M, Miyaji A, Tsuji K, Nakajo T. Catalytic chemistry of supported heteropolyacids and their applications as solid acids to industrial processes. Catal Surv Asia. 2008; 12: 101-113. https://doi.org/10.1007/s10563-008-9043-7
[2] Chikin AI, Chernyak AV, Jin Z, Naumova YS, Ukshe AE, Smirnova NV, Volkov VI, Dobrovolsky YA. Mobility of protons in 12-phosphotungstic acid and its acid and neutral salts. J Solid State Electrochem. 2012; 16: 2767-2775. https://doi.org/10.1007/s10008-012-1687-6
[3] Kourasi AM, Wills RGA, Shah AA, Walsh FC. Heteropolyacids for fouel cell applications. Electrochim Acta. 2014; 127: 454-466. https://doi.org/10.1016/j.electacta.2014.02.006
[4] Imofeeva MN, Maksimov GM, Likholobov VA. Acidity of solutions of heteropoly acids with various structures and compositions. Kinetic Catal. 2001; 42: 30-34. https://doi.org/10.1023/A:1004892411282
[5] Kima JD, Hayashi S, Mori T, Honma I. Fast proton conductor under anhydrous condition synthesized from 12-phosphotungstic acid and ionic liquid. Electrochim Acta. 2007; 53: 963-967. https://doi.org/10.1016/j.electacta.2007.08.009
[6] Schneea J, Devredb F, Gaigneauxb EM, Vimonta A, Assessing the dispersion of supported H3PW12O40 catalysts: No longer a hurdle thanks to in situ IR upon pyridine adsorption. Appl Catal. 2019; 578: 116-121. https://doi.org/10.1016/j.apcata.2019.03.022
[7] Brown GM, Noe-Spirlet MR, Busing WR, Levy HA. Dodecatungstophosphoric acid hexahydrate, (H5O2+)3(PW12O403-). The true structure og Keggins pentahydrate from single-crystal X-ray and neutron diffraction data. Acta Cryst. 1977; B33: 1038-1046. https://doi.org/10.1107/S0567740877005330
[8] Spirlet MR, Busing WR. Dodecatungstophosphoric acid-21-water by neutron diffraction. Acta Cryst. 1978; 34(3): 907-910. https://doi.org/10.1107/S0567740878004306
[9] Kremenović A, Spasojević-de Biré A, Dimitrijević R, Sciau P., Mioč UB, Colomban Ph. Keggin’s ion structural modification and expansion of dodecatungstophosphoric acid hexahydrate induced by temperature treatment: In situ X-ray powder diffraction and Raman investigations. Solid State Ion. 2000; 132: 39-53. https://doi.org/10.1016/S0167-2738(00)00727-X
[10] Davidović M, Čajkovski T, Colomban Ph, Mioč UB, Likar-Smiljanić V, Čajkovski D, Biljić R, Nedić Z. The influence of monovalent and bivalent cations on the electrical properties of 12-tungstophosphoric acid salts. Solid State Ion. 2005; 176: 2881-2885. https://doi.org/10.1016/j.ssi.2005.09.020
[11] Kulesza PJ, Rutkowska IA, Janiszewska C, Noto VD, Vezzu K, Negro E. Development and characterization of polyoxometallate-based systems for aqueous redox flow batteries. Meet Abstr. 2022; MA2022-01: 1999. https://doi.org/10.1149/MA2022-01481999mtgabs
[12] Ammam M, Fransaer J. Ionic liquid-heteropolyacid: Synthesis, characterization, and supercapacitor study of films deposited by electrophoresis. J Electrochem Soc. 2011; 158: A14. https://doi.org/10.1149/1.3507254
[13] Mioč UB, Dimitrijević RŽ, Davidović M, Nedić ZP, Mitrović MM, Colomban Ph. Thermally inducted phase transformations of 12-tungstophosphoric acid 29-hydrate:synthesis and characterization of PW8O26-type bronzes. J Mater Sci. 1994; 29: 3705-3718. https://doi.org/10.1007/BF00357338
[14] Dimitrijević RŽ, Colomban Ph, Mioč UB, Nedić Z, Todorović MR, Tjapkin N, Davidović M. Synthesis, conductivity and structural characterization of phosphorous bronzes originating from heteropolyacids. Relation with similar proton containing phases. Solid State Ion. 1995; 77: 250-256. https://doi.org/10.1016/0167-2738(94)00310-O
[15] Maksimović TV, Maksimović JP, Joksović LjG, Nedić ZP, Pagnacco MC. Oscilatorna reakcija kao sistem detektor za dopirane i nedopirane fosfat-volframove bronze. Hem Ind. 2018; 72(5): 275-283. (in Serbian) https://doi.org/10.2298/HEMIND180402018M
[16] Sweedler AR, Raub ChJ, Matthias BT. Superconductivity of the alkali tungsten bronzes. Phys Lett. 1965; 15(2): 108-109. https://doi.org/10.1016/0031-9163(65)91292-8
[17] Brusetti R, Bordet P, Bossy J, Schober H, Eibl S. Superconductivity in the tungsten bronze RbxWO3 (0.20⩽ x⩽ 0.33) in connection with its structure, electronic density of states, and phonon density of states. Phys Rev. 2007; B76: 174511. https://doi.org/10.1103/PhysRevB.76.174511
[18] Yoon S, Jo C, Noh SY, Lee CW, Song H, Lee J. Development of a high-performance anode for lithium ion batteries using novel ordered mesoporous tungsten oxide materials with high electrical conductivity. PCCP. 2011; 13: 11060-11066. https://doi.org/10.1039/C1CP20940J
[19] Wasserman K, Pope MT, Salmen M, Dann JN, Lunk HJ. Thermal degradation of polyoxotungstates-an effective method for the preparation of tungsten bronzes. J Solid State Chem. 2000; 149: 378-383. https://doi.org/10.1006/jssc.1999.8556
[20] Dong X, Lu Y, Wu Z, Liu X, Tong Y. Photochromic hierarchical (NH4)xWO3 nanocrystals with bronze tunnel structure for energy-saving windows. Chem Eng J. 2023; 477: 147064. https://doi.org/10.1016/j.cej.2023.147064
[21] Acković J, Micić R, Nedić Z, Petrović T, Senćanski J, Pagnacco M, Tančić P. Synthesis, characterization and electrochemical properties of iron doped phosphate tungsten heteropoly acid (Fe-PWA) and its bronze (Fe-PWB): Comparative study. Sci Sinter. 2024; 56: 367-380. https://doi.org/10.2298/SOS230812053A
[22] Pagnacco M, Marković S, Potočnik J, Krstić V, Tančić P, Mojović M, Mojović Z. The influence of electrode constituents on hydrogen evolution reaction on phosphate W-and Mo bronze based electrodes. J Electrochem Soc. 2022; 169(10): 106508. https://doi.org/10.1149/1945-7111/ac96ab
[23] Maksimović JP, Maksimović TV, Nedić ZP, Pagnacco MC. The minor influence of calcium doped phosphate tungsten bronze on the Briggs-Rauscher reaction dynamics. Contemp Mater. 2018; 184-189. https://doi.org/10.7251/COMEN1802184M
[24] Maksimović TV, Maksimović JP, Tančić PI, Potkonjak NI, Nedić ZP, Joksović LjG, Pagnacco MC. A possible connection between phosphate tungsten bronzes properties and Briggs-Rauscher oscillatory reaction response. Sci Sinter. 2021; 53: 223. https://doi.org/10.2298/SOS2102223M
[25] Maksimović T, Tančić P, Maksimović J, Mara D, Ilić M, Van Deun R, Joksović Lj, Pagnacco M. Novel cerium and praseodymium doped phosphate tungsten bronzes: Synthesis, characterization, the behavior in the Briggs-Rauscher reaction and photoluminescence properties. Opt Mater. 2023; 143: 114125. https://doi.org/10.1016/j.optmat.2023.114125
[26] Vujković M, Nedić Z, Tančić P, Aleksić OS, Nikolić MV, Mioč U, Mentus S. Electrochemical lithiation/delithiation kinetics and capacity of phosphate tungsten bronze and its chemically pre-lithiated derivatives aqueous solutions. J Mater Sci. 2016; 51: 2481-2489. https://doi.org/10.1007/s10853-015-9560-5
[27] Rodriguez-Carvajal, J. Program Fullprof (Computer software). In Proceedings of the Abstract of 15th Conference of International Union of Crystallography, Satellite Meeting on Powder Diffraction, Toulouse, France, July 16-19th 1990; p. 127.
[28] Tančić P, Dimitrijević R, Poznanović M, Pačevski A, Sudar S. Crystal structure and chemical composition of ludwigite from Vranovac ore deposit (Boranja Mountain, Serbia). Acta Geol Sin-Engl. 2012; 86(6): 1524-1538. https://doi.org/10.1111/1755-6724.12020
[29] Tančić P, Kremenović A, Vulić P. Structural dissymmetrization of optically anisotropic Grs64±1Adr36±1Sps2 grandite from Meka Presedla (Kopaonik Mt., Serbia). Powder Diffr. 2020; 35(1): 7-16. https://doi.org/10.1017/S0885715619000897
[30] Tančić P, Kremenović A. Rietveld crystal structure refinement of a natural rhombohedral grossular-andradite garnet from Serbia. Geol Q. 2022; 66(7): 1639. http://dx.doi.org/10.7306/gq.1639
[31] Mioč U, Davidović M, Tjapkin N, Colomban Ph, Novak A. Equilibrium of the protonic species in hydrates of some heteropolyacids at elevated temperatures. Solid State Ion. 1991; 46: 103-109. https://doi.org/10.1016/0167-2738(91)90136-Y
[32] Ratajczak H, Barnes AJ, Bielański A, Lutz HD, Müller A, Pope MT. Vibrational Spectroscopy of Heteropoly Acids. In: Pope, MT, Müller A. (eds) Polyoxometalate Chemistry From Topology via Self-Assembly to Applications. Springer Dordrecht. 2001; 101-116. https://doi.org/10.1007/0-306-47625-8_8
[33] Jose da Silva M, Macedo de Oliveira C. Catalysis by Keggin heteropolyacid salts. Curr Catal. 2018; 7: 26-34. https://doi.org/10.2174/2211544707666171219161414
[34] Rocchiccioli-Deltcheff C, Fournier M, Franck R, Thouvenot R, Vibrational investigations of polyoxometalates. 2. Evidence for anion-anion interactions in molybdenum(VI) and tungsten(VI) compounds related to the keggin structure. Inorg Chem. 1983; 22: 207-216. https://doi.org/10.1021/ic00144a006
[35] Martinez-de la Cruz A, Rodriguez FEL, Rodriguez JI. Electrochemical lithium insertion in the phosphate tungsten bronze P8W12O52. Solid State Ion. 2005; 176: 2625-2630. https://doi.org/10.1016/j.ssi.2005.08.009
[36] Martínez-de la Cruz A, Rodríguez FEL. Electrochemical lithium insertion in (PO2)4(WO3)2m (2≤m≤10): Relation among the electrochemical insertion process and structural features. Electrochim Acta. 2009; 54: 3176-3183. https://doi.org/10.1016/j.electacta.2008.11.056
[37] Wang E, Greenblatt M. Lithium and sodium insertion reactions of phosphate tungsten bronzes. J Solid State Chem. 1987; 68: 38-44. https://doi.org/10.1016/0022-4596(87)90282-9