Phase transformations kinetics in barium titanate synthesis by mechanochemical processing Original scientific paper
Main Article Content
Abstract
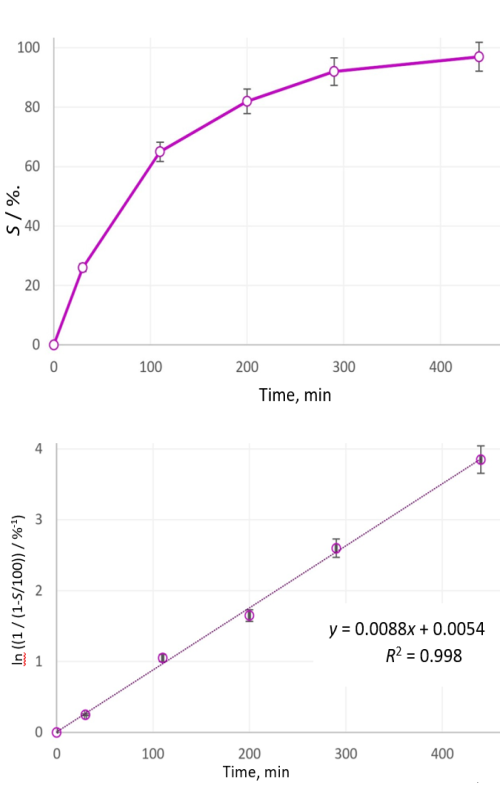
This article presents the research results on a dry mechanochemical synthesis of barium titanate at a low temperature in which the reaction model and kinetics were determined during the activation of a powder mixture of titanium dioxide and barium oxide. The solid-state reaction achieved high degree of conversion (0.99). Successive analyses were conducted throughout the reaction, revealing the presence of both the starting powders and newly formed intermediate compounds. Phase transformations were monitored via X-ray diffraction, allowing the dynamics of the synthesis to be characterized. It was established that, for the given system, 440 min of mechanical activation in a high-energy vibration mill was required to complete the neutralization reaction and produce barium titanate. The reaction mixture composition was tracked by sampling at five intervals, confirming the presence of intermediate compounds and mapping the reaction pathway from the initial barium and titanium oxides to the final BaTiO3 product.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-66/2024-03/200023;451-03-65/2024-03/200131;451-03-66/2024-03/200026
References
[1] Vijatović M, Bobić J, Stojanović B. History and Challenges of Barium Titanate: Part I. Sci Sinter. 2008; 10L: 155-165 https://doi.org/10.2298/SOS0802155V
[2] Prado L, Resende N, Silva R, Egues S, Salazar-Banda G. Influence of the synthesis method on the preparation of barium titanate nanoparticles. Chem Eng Process: Process Intensif. 2016; 103: 12-20 https://doi.org/10.1016/j.cep.2015.09.011
[3] Kubota K, Seo T, Koide K, Hasegawa Y, Ito H. Olefin-accelerated solid-state C-N cross-coupling reactions using mechanochemistry. Nat Commun. 2019; 10: 111 https://doi.org/10.1038/s41467-018-08017-9
[4] Zhang X, Zhou KS, Liu M, Deng CM, Deng CG,Deng ZQ. Adsorbability and spreadability of calcium-magnesium-alumino-silicate (CMAS) on Al-modified 7YSZ thermal barrier coating. Ceram Int. 2016; 42(16): 19349-19356 https://doi.org/10.1016/j.ceramint.2016.09.106
[5] Oleynikov NN, Tretyakov YD, Shumyantzev AV. Concerning the activation energy of solid state reactions. J Solid State Chem. 1974;11 (4):340-343 https://doi.org/10.1016/S0022-4596(74)80039-3
[6] Gomes W. Definition of Rate Constant and Activation Energy in Solid State Reactions. Nature. 1961;192: 865-866 https://doi.org/10.1038/192865a0
[7] Behrens M. Meso-and nano-structuring of industrial Cu/ZnO/(Al2O3) catalysts. J Catal.2009;267 (1):24-29 https://doi.org/10.1016/j.jcat.2009.07.009
[8] Criado JM. On the determination of the activation energy of solid-state reactions from the maximum reaction rate of isothermal runs. J Therm Anal. 1981; 21: 155-157 https://doi.org/10.1007/BF01913708
[9] Lewis GN. The atom and the molecule. J Am Chem Soc. 1916; 38 (4): 762-785 https://doi.org/10.1021/ja02261a002
[10] Avvakumov EG. Mekhanicheskiemetodyaktivaciikhimicheskihprocesov.2rd ed., Novosibirsk, Nauka; 1986 (in Russian)
[11] Boldyrev VV. Mechanochemistry and mechanical activation of solids. Solid State Ion. 1993; 63-65:537-543 https://doi.org/10.1016/0167-2738(93)90157-X
[12] Lazarević ZŽ, Bobić J, Romčević NŽ, Paunović N, Stojanović BD. Study of Barium Bismuth Titanate Prepared by Mechanochemical Synthesis. Sci Sinter.2009; 41: 329-335 https://doi.org/10.2298/SOS0903329L
[13] Obradović N, Filipović S, Pavlović V, Mitrić M, Marković S, Mitić V,Đorđević N, Ristić MM. Isothermal sintering of barium-zinc-titanate ceramics. Ceram Int. 2011; 37(1): 21-27 https://doi.org/10.1016/j.ceramint.2010.07.001
[14] Zdujić M, Poleti D, Jovalekić C, Karanović L. The evolution of structure induced by intensive milling in the system 2Bi2O3•3TiO2. J Non-Cryst Solids. 2006;352 (28-29): 3058-3068 https://doi.org/10.1016/j.jnoncrysol.2006.03.072
[15] Buscaglia V, Randall CA. Size and scaling effects in barium titanate. J Eur Ceram. 2020; 40: 3744-3758 https://doi.org/10.1016/j.jeurceramsoc.2020.01.021
[16] Reynolds GJ. Electrical Properties of Thin-Film Capacitors Fabricated Using High Temperature Sputtered Modified Barium titanate. Materials. 2012; 5: 644-660 https://doi.org/10.3390/ma5040644
[17] Wei X, Liu Y, Zhao D, Ge SS. 3D printing of piezoelectric barium tinatate with high density form milled powders. J Eur Ceram. 2020; 43 (8):3297-3306 https://doi.org/10.1016/j.jeurceramsoc.2020.06.021
[18] Brzozowski E, Castro MS. Synthesis of barium titanate improved by modications in the kinetics of the solid state reaction. J Eur Ceram. 2000;20: 2347-2351 https://doi.org/10.1016/S0955-2219(00)00148-5
[19] Kozawa T, Onda A, Yanagisawa K. Accelerated formation of barium titanate by solid-state reaction in water vapour atmosphere. J Eur Ceram. 2009; 29: 3259-3264 https://doi.org/10.1016/j.jeurceramsoc.2009.05.031
[20] Apaydin F, Parlak TT, Yıldız K. Low temperature formation of barium titanate in solid state reaction by mechanical activation of BaCO3 and TiO2. Mater Res Express. 2019; 6: 126330 https://doi.org/10.1088/2053-1591/ab6c0d
[21] Ashiri R. On the solid-state formation of BaTiO3 nanocrystals from mechanically activated BaCO3 and TiO2 powders: innovative mechanochemical processing, the mechanism involved, and phase and nanostructure evolutions. RSC Adv. 2016; 6: 17138 https://doi.org/10.1039/C5RA22942A
[22] Ziegmann A, Schubert DW. Influence of the particle size and the filing degree of barium titanate filled silicone elastomers used as potential dielectric elastomers on the mechanical properties and the crosslinking density. MaterToday Commun. 2018; 14: 90-98 https://doi.org/10.1016/j.mtcomm.2017.12.013
[23] Uttam R, Yadav N, Kumar S, Dhar R. Strengthening of columnar hexagonal phase of a room temperature discotic liquid crystalline material by using ferroelectric barium titanate nanoparticles. J Mol Liq. 2019; 294: 111609 https://doi.org/10.1016/j.molliq.2019.111609
[24] Gu L, Li T, Xu Y, Sun C, Yang Z, Zhu D, Chen D. Effects of the particle size of BaTiO3 fillers on fabrication and dielectric properties of BaTiO3/Polymer/Al films for capacitor energy-storage application. Materials. 2019;12: 439 https://doi.org/10.3390/ma12030439
[25] Binhayeeniyi N, Sukwisute P, Nawae S, Muensit N. Energy conversion capacity of barium zirconatetitanate. Materials. 2020; 13: 315 https://doi.org/10.3390/ma13020315
[26] Polley C, Distler T, Detsch R, Lund H, Springer A, Boccaccini AR, Seitz H. 3D Printing of Piezoelectric Barium Titanate-Hydroxyapatite Scaffolds with Interconnected Porosity for Bone Tissue Engineering. Materials.2020; 13: 1773 https://doi.org/10.3390/ma13071773
[27] Jelinek M, Vanek P, Tolde Z. Buixaderas E, Kocourek T, Studnicka V, Drahokoupil J, Petzelt J, Remsa J, Tyunina M. PLD preparet bioactive BaTiO3 films on TiNb implants. Mater Sci Eng. C. 2017; 70: 334-339 https://doi.org/10.1016/j.msec.2016.08.072
[28] Stojanović BD, Simoes AZ, Paiva-Santos CO, Jovalekić C, Mitic VV, Varela JA. Mechanochemical synthesis of barium titanate. J Eur Ceram. 2005; 25: 1985-1989 https://doi.org/10.1016/j.jeurceramsoc.2005.03.003
[29] Đorđević NG, Matijašević SD, Mihajlovic SR, Stojanović JN, Radulović AM, Savić LjB. The Effect of Particle Size on the Crystallization LiGe2(PO4)3 Phase from Glass. Sci Sinter. 2025; 57: 43-52 https://doi.org/10.2298/SOS231111064D
[30] Vidojkovic VM. Proučavanje mehanizma i kinetike mehanohemijske sinteze neorganskih soli kod reakcija neutralizacije, Doktorska disertacija, Univerzitet u Beogradu, 2001.
[31] Roine A. HSC Chemistry for Windows Chemical Reaction and Equilibrium Software with Extensive Database. Version 2.03, Outokumpu Research Oy, Pori, Finland 1994
[32] Ptaček P, Opravil T, Šoukal F. Introducing the Effective Mass of Activated Complex and the Discussion on the Wave Function of this Instanton. IntechOpen, London UK, 2018. https://doi.org/10.5772/intechopen.70734
[33] Đorđević N, Vlahović M, Mihajlović S. X-ray structural analysis of the BaO and TiO2 starting compounds and initial mechanochemical activation. Undeground Mining Engineering.2023; 42: 37-46 https://doi.org/10.5937/podrad2342037Q
[34] Botta PM, Aglietti EF, López JMP. Mechanochemical effects on the kinetics of zinc titanate formation. J Mater Sci. 2004; 39:5195-5199 https://doi.org/10.1023/B:JMSC.0000039209.48875.25
[35] Revellame ED, Fortela DL, Sharp W, Hernandez R, Zappi ME. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean Eng Technol. 2020; 1: 100032 https://doi.org/10.1016/j.clet.2020.100032
[36] Dondur V. Hemijska kinetika, Beograd, Fakultet za fizičku-hemiju; 1992 (in Serbian)