Uticaj dodatka poli(dialildimetilamonijum hlorida) na kinetiku očvršćavanja urea-formaldehidnog adheziva za ploče iverice Naučni rad
Glavni sadržaj članka
Apstrakt
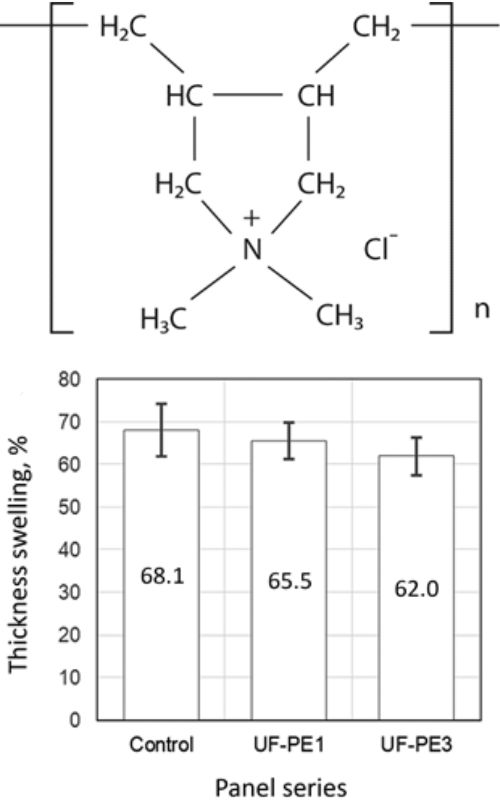
U ovom radu ispitan je uticaj dodatka poli(dialildimetilamonijum hlorida) (PDDA) na performanse urea-formaldehidnog (UF) adheziva. U tom cilju pripremljene su tri serije UF adheziva: bez dodatka PDDA i sa dodatkom PDDA od 1 i 3 % suve supstance po masi suve supstance adheziva. Dodatak PDDA smanjio je debljinsko bubrenje uzoraka eksperimentlano dobijenih ploča iverica, dok je zatezna čvrstoća upravno na površinu ploče značajno povećana tek pri dodatku PDDA od 3 %. Metoda diferencijalne skeirajuće kalorimetrije (engl. differential scanning calorimetry, DSC) primenjena je u cilju ispitivanja uticaja dodatka PDDA na kinetiku očvršćavanja UF adheziva. DSC merenja sprovedena su u dinamičkom režimu korišćenjem različitih brzina zagrevanja (5, 10 i 20 °C∙min−1). Povećanje koncentracije PDDA uticalo je na smanjenje vrednosti energije aktivacije očvršćavanja UF adheziva, izračunate po opštem Kisindžerovom modelu. Zavisnost energije aktivacije u odnosu na stepen konverzije određena je za sva tri ispitivana adhezivna sistema korišćenjem izo-konverzionih modela (Kissinger-Akahira-Sunose i Friedman), što je omogućilo detaljniji uvid u uticaj PDDA na tok reakcije očvršćavanja UF adheziva.
Detalji članka
Broj časopisa
Rubrika

Ovaj rad je pod Creative Commons Aуторство-Nekomercijalno-Bez prerade 4.0 Internacionalna licenca.
Kada je rukopis prihvaćen za objavlјivanje, autori prenose autorska prava na izdavača. U slučaju da rukopis ne bude prihvaćen za štampu u časopisu, autori zadržavaju sva prava.
Na izdavača se prenose sledeća prava na rukopis, uklјučujući i dodatne materijale, i sve delove, izvode ili elemente rukopisa:
- pravo da reprodukuje i distribuira rukopis u štampanom obliku, uklјučujući i štampanje na zahtev;
- pravo na štampanje probnih primeraka, reprint i specijalnih izdanja rukopisa;
- pravo da rukopis prevede na druge jezike;
- pravo da rukopis reprodukuje koristeći fotomehanička ili slična sredstva, uklјučujući, ali ne ograničavajući se na fotokopiranje, i pravo da distribuira ove kopije;
- pravo da rukopis reprodukuje i distribuira elektronski ili optički koristeći sve nosioce podataka ili medija za pohranjivanje, a naročito u mašinski čitlјivoj/digitalizovanoj formi na nosačima podataka kao što su hard disk, CD-ROM, DVD, Blu-ray Disc (BD), mini disk, trake sa podacima, i pravo da reprodukuje i distribuira rukopis sa tih prenosnika podataka;
- pravo da sačuva rukopis u bazama podataka, uklјučujući i onlajn baze podataka, kao i pravo prenosa rukopisa u svim tehničkim sistemima i režimima;
- pravo da rukopis učini dostupnim javnosti ili zatvorenim grupama korisnika na osnovu pojedinačnih zahteva za upotrebu na monitoru ili drugim čitačima (uklјučujući i čitače elektonskih knjiga), i u štampanoj formi za korisnike, bilo putem interneta, onlajn servisa, ili putem internih ili eksternih mreža.
Kako citirati
Reference
Li H, Wang L, Sheng K, Zou L, Ye B. Highly sensitive determination of esculetin on TiO2-NPs-coated poly(diallyl¬dimethyl¬amm-onium chloride)-functionalized graphene modified electrode. Talanta. 2016;161:838-846 https://doi.org/10.1016/j.talanta.2016.09.050
Wang B, Okoth OK, Yan K, Zhang J. A highly selective electrochemical sensor for 4-chlorophenol determination based on molecularly imprinted polymer and PDDA-functionalized graphene. Sensors Actuators, B Chem. 2016;236(236):294-303 https://doi.org/10.1016/j.snb.2016.06.017
Okoth OK, Yan K, Liu L, Zhang J. Simultaneous Electrochemical Determination of Paracetamol and Diclofenac Based on Poly(diallyldimethylammonium chloride) Functionalized Graphene. Electroanalysis. 2016;28(1):76-82 https://doi.org/10.1002/elan.201500360
Lu J, Liu Y, Liu X, Lu X, Liu X. Construction of a highly sensitive NADH sensing platform based on PDDA-rGO nanocomposite modified electrode. Ionics (Kiel). 2016;22(11):2225-2233 https://doi.org/10.1007/s11581-016-1753-7
Borowiec J, Yan K, Tin C-C, Zhang J. Synthesis of PDDA Functionalized Reduced Graphene Oxide Decorated with Gold Nanoparticles and Its Electrochemical Response toward Levofloxacin. J Electrochem Soc. 2015;162(3):H164-H169 https://doi.org/10.1149/2.0811503jes
Li F, Yang Q, Qiu F, Liu Y. Modification of superparamagnetic iron oxide nanoparticles with poly(diallyldimethylammonium chloride) at air atmosphere. Polym Adv Technol. 2016;27(11):1530-1534 https://doi.org/10.1002/pat.3834
Cho E, Won J. Novel composite membrane coated with a poly(diallyldimethylammonium chloride)/urushi semi-interpenetrating polymer network for non-aqueous redox flow battery application. J Power Sources. 2016;335:12-19 https://doi.org/10.1016/j.jpowsour.2016.10.020
Zhang J, Qiao J, Jiang G, Liu L, Liu Y. Cross-linked poly(vinyl alcohol)/poly (diallyldimethylammonium chloride) as anion-exchange membrane for fuel cell applications. J Power Sources. 2013;240:359-367 https://doi.org/10.1016/j.jpowsour.2013.03.162
Pandit S, Khilari S, Bera K, Pradhan D, Das D. Application of PVA-PDDA polymer electrolyte composite anion exchange membrane separator for improved bioelectricity production in a single chambered microbial fuel cell. Chem Eng J. 2014;257:138-147 https://doi.org/10.1016/j.cej.2014.06.077
Lin Z, Renneckar S, Hindman DP. Nanocomposite-based lignocellulosic fibers 1. Thermal stability of modified fibers with clay-polyelectrolyte multilayers. Cellulose. 2008;15(2):333-346 https://doi.org/10.1007/s10570-007-9188-y
Pillai K V., Renneckar S. Dynamic mechanical analysis of layer-by-layer cellulose nanocomposites. Ind Crops Prod. 2016;93:267-275 https://doi.org/10.1016/j.indcrop.2016.02.037
Zhang L, Chen H, Sun J, Shen J. Layer-by-layer deposition of poly(diallyldimethylammonium chloride) and sodium silicate multilayers on silica-sphere-coated substrate-facile method to prepare a superhydrophobic surface. Chem Mater. 2007;19(4):948-953 https://doi.org/10.1021/cm062535i
Sadeghi B, Pourahmad A. Synthesis of silver/poly (diallyldimethylammonium chloride) hybride nanocomposite. Adv Powder Technol. 2011;22(5):669-673 https://doi.org/10.1016/j.apt.2010.10.001
Huang J, Liu X, Thormann E. Surface Forces between Highly Charged Cationic Polyelectrolytes Adsorbed to Silica: How Control of pH and the Adsorbed Amount Determines the Net Surface Charge. Langmuir. 2018;34(25):7264-7271 https://doi.org/10.1021/acs.langmuir.8b00909
Zhang H, Zhao C, Li Z, Li J. The fiber charge measurement depending on the poly-DADMAC accessibility to cellulose fibers. Cellul 2015 231. 2015;23(1):163-173 https://doi.org/10.1007/s10570-015-0793-x
McLean D, Agarwal V, Stack K, Horne H, Richardson D. Synthesis of guar gum-graft-poly (acrylamide-co-diallyldimethylammonium chloride) and its application in the pulp and paper industry. BioResources. 2011;6(4):4168-4180 https://bioresources.cnr.ncsu.edu/resources/synthesis-of-guar-gum-graft-polyacrylamide-co-diallyldimethylammonium-chloride-and-its-application-in-the-pulp-and-paper-industry/
EN 827: Adhesives - Determination of conventional solids content and constant mass solids. 2005
EN 12092: Adhesives - Determination of viscosity. 2001
EN 1245: Adhesives - Determination of pH - Test method. 1998
EN 322: Wood-based panels - Determination of moisture content. 1993
EN 323: Wood-based panels - Determination of density. 1993
EN 317: Particleboards and fibreboards - Determination of swelling in thickness after. 1993
EN 319: Particleboards and fibreboards - Determination of tensile strength perpendicular to the plane of the board. 1993
Popović M, Popović J, Điporovic-Momčilović M, Vukić N, Budinski-Simendić J, Gavrilović-Grmuša I, Hamid F. The curing behavior of urea-formaldehyde adhesive in the presence of chemically treated narrow-leaved ash. Zast Mater. 2019;60(1):64-69 https://doi.org/10.5937/zasmat1901064P
Vyazovkin S. Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem. 2001;22(2):178-183 https://doi.org/10.1002/1096-987X(20010130)22:2<178::AID-JCC5>3.0.CO;2-%23
Kandelbauer A, Wuzella G, Mahendran A, Taudes I, Widsten P. Model-free kinetic analysis of melamine-formaldehyde resin cure. Chem Eng J. 2009;152(2-3):556-565 https://doi.org/10.1016/j.cej.2009.05.027
Sbirrazzuoli N, Vincent L, Mija A, Guigo N. Integral, differential and advanced isoconversional methods. Complex mechanisms and isothermal predicted conversion-time curves. Chemom Intell Lab Syst. 2009;96(2):219-226 https://doi.org/10.1016/j.chemolab.2009.02.002
Zhang C, Binienda WK, Zeng L, Ye X, Chen S. Kinetic study of the novolac resin curing process using model fitting and model-free methods. Thermochim Acta. 2011;523(1-2):63-69 https://doi.org/10.1016/j.tca.2011.04.033
Starink MJ. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404(1-2):163-176 https://doi.org/10.1016/S0040-6031(03)00144-8
EN 312: Particleboards - Specifications. 2010
Tischer S, Börnhorst M, Amsler J, Schoch G, Deutschmann O. Thermodynamics and reaction mechanism of urea decomposition. Phys Chem Chem Phys. 2019;21(30):16785-16797 https://doi.org/10.1039/C9CP01529A
Gao J, Zhao M, Qin J. Curing Kinetics of o-Cresol-formaldehyde Epoxy Resin/3-Methyl-tetrahydrophthalic Anhydride/Organic-Montmorillonite Nanocomposite by Isoconversional Methods. Iran Polym J. 2006;15(5):425-432 https://www.sid.ir/en/journal/ViewPaper.aspx?id=103253