Diferencijalna skenirajuća kalorimetrijska ispitivanja faznopromenljivog kompozita eritritol – natrijum hlorid za primenu u skladištenju toplotne energije Naučni rad
Glavni sadržaj članka
Apstrakt
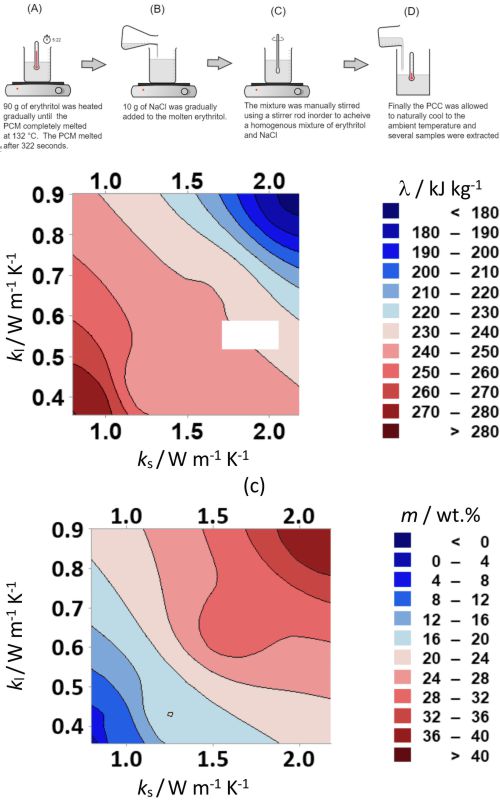
Zbog niske toplotne provodljivosti organskih faznopromenljivih materijala (phase change materials-PCM), njihova primena u sistemima za skladištenje toplotne energije zahteva korišćenje odgovarajućih tehnika radi poboljšanje prenosa toplote. Cilj ove studije je bio poboljšanje toplotne provodljivosti eritritola kao faznopromenljivog materijala, korišćenjem natrijum-hlorida (NaCl) kao aditiva, tako da se materijal može primeniti za sisteme za kuvanje na pari kada je integrisan sa solarnim paraboličnim kolektorima. Eritritol-NaCl smeše su sintetisane metodom topljenja, a ključne fizičko-hemijske osobine smeše su određene primenom diferencijalne skenirajuće kalorimetrije (DSC) u kombinaciji sa termo-gravimetrijskom analizom (TGA). Resultati pokazuju da je došlo do značajnog poboljšanja toplotne provodljivosti eritritola sa dodatkom NaCl u odnosu na čist eritritol. Takođe, termičko ponašanje materijala ukazuje na to da je pogodan za kuvanje na pari. Razvijeni matematički modeli zasnovani na eksperimentalnim rezultatima u ovoj studiji, mogu se potencijalno koristiti za dalja istraživanja kompozita eritritol-NaCl.
Detalji članka
Broj časopisa
Rubrika

Ovaj rad je pod Creative Commons Aуторство-Nekomercijalno-Bez prerade 4.0 Internacionalna licenca.
Kada je rukopis prihvaćen za objavlјivanje, autori prenose autorska prava na izdavača. U slučaju da rukopis ne bude prihvaćen za štampu u časopisu, autori zadržavaju sva prava.
Na izdavača se prenose sledeća prava na rukopis, uklјučujući i dodatne materijale, i sve delove, izvode ili elemente rukopisa:
- pravo da reprodukuje i distribuira rukopis u štampanom obliku, uklјučujući i štampanje na zahtev;
- pravo na štampanje probnih primeraka, reprint i specijalnih izdanja rukopisa;
- pravo da rukopis prevede na druge jezike;
- pravo da rukopis reprodukuje koristeći fotomehanička ili slična sredstva, uklјučujući, ali ne ograničavajući se na fotokopiranje, i pravo da distribuira ove kopije;
- pravo da rukopis reprodukuje i distribuira elektronski ili optički koristeći sve nosioce podataka ili medija za pohranjivanje, a naročito u mašinski čitlјivoj/digitalizovanoj formi na nosačima podataka kao što su hard disk, CD-ROM, DVD, Blu-ray Disc (BD), mini disk, trake sa podacima, i pravo da reprodukuje i distribuira rukopis sa tih prenosnika podataka;
- pravo da sačuva rukopis u bazama podataka, uklјučujući i onlajn baze podataka, kao i pravo prenosa rukopisa u svim tehničkim sistemima i režimima;
- pravo da rukopis učini dostupnim javnosti ili zatvorenim grupama korisnika na osnovu pojedinačnih zahteva za upotrebu na monitoru ili drugim čitačima (uklјučujući i čitače elektonskih knjiga), i u štampanoj formi za korisnike, bilo putem interneta, onlajn servisa, ili putem internih ili eksternih mreža.
Kako citirati
Reference
Indora S, Kandpal TC. Institutional cooking with solar energy: A review. Renew Sustain Energ Rev 2018;84:131–54. https://doi.org/https://doi.org/10.1016/j.rser.2017.12.001
Motwani K, Patel J. Cost analysis of solar parabolic trough collector for cooking in Indian hostel–a case study. Int J Ambient Energy 2019;0(0):1–17. https://doi.org/10.1080/01430750.2019.1653968
Kalogirou SA. Solar thermal collectors and applications. Prog Energ Combust 2004;30(3):231-295. https://doi.org/10.1016/j.pecs.2004.02.001
Ravi Kumar K, Krishna Chaitanya NVV, Sendhil Kumar N. Solar thermal energy technologies and its applications for process heating and power generation. J Clean Prod 2021;282:125296. https://doi.org/10.1016/j.jclepro.2020.125296
Koçak B, Fernandez AI, Paksoy H. Review on sensible thermal energy storage for industrial solar applications and sustainability aspects. Sol Energy 2020;209:135–69. https://doi.org/10.1016/j.solener.2020.08.081
Akba T, Baker D, Yazıcıoğlu AG. Modeling, transient simulations and parametric studies of parabolic trough collectors with thermal energy storage. Sol Energy 2020;199:497–509. https://doi.org/10.1016/j.solener.2020.01.079
Ghazouani M, Bouya M, Benaissa M, Anoune K, Ghazi M. Thermal energy management optimization of solar thermal energy system based on small parabolic trough collectors for bitumen maintaining on heat process. Sol Energy 2020;211:1403–21. https://doi.org/10.1016/j.solener.2020.10.074
Biencinto M, Bayón R, González L, Christodoulaki R, Rojas E. Integration of a parabolic-trough solar field with solid-solid latent storage in an industrial process with different temperature levels. Appl Therm Eng 2021;184:116263. https://doi.org/10.1016/j.applthermaleng.2020.116263
Shchukina EM, Graham M, Zheng Z, Shchukin DG. Nanoencapsulation of phase change materials for advanced thermal energy storage systems. Chem Soc Rev 2018;47(11):4156–75. https://doi.org/10.1039/c8cs00099a
Sutjahja IM, Rahman A, Putri RA, et al. Electrofreezing of the phase-change material CaCl2·6H2O and its impact on supercooling and the nucleation time. Hem Ind 2019;73(6):363–74. https://doi.org/10.2298/HEMIND190803034S
Jahanpanah M, Sadatinejad SJ, Kasaeian A, Jahangir MH, Sarrafha H. Experimental investigation of the effects of low-temperature phase change material on single-slope solar still. Desalination 2021;499:114799. https://doi.org/10.1016/j.desal.2020.114799
Atinafu DG, Ok YS, Kua HW, Kim S. Thermal properties of composite organic phase change materials (PCMs): A critical review on their engineering chemistry. Appl Therm Eng 2020;181:115960. https://doi.org/10.1016/j.applthermaleng.2020.115960
Magendran SS, Khan FSA, Mubarak NM, et al. Synthesis of organic phase change materials (PCM) for energy storage applications: A review. Nano-Structures and Nano-Objects 2019;20:100399. https://doi.org/10.1016/j.nanoso.2019.100399
Singh P, Sharma RK, Ansu AK, Goyal R. Study on thermal properties of organic phase change materials for energy storage. Mater Today Proc 2020;28(4):2353–7. https://doi.org/10.1016/j.matpr.2020.04.640
Sharma RK, Ganesan P, Tyagi V V., Metselaar HSC, Sandaran SC. Developments in organic solid-liquid phase change materials and their applications in thermal energy storage. Energy Convers Manag 2015;95:193–228. https://doi.org/10.1016/j.enconman.2015.01.084
Atinafu DG, Dong W, Huang X, Gao H, Wang G. Introduction of organic-organic eutectic PCM in mesoporous N-doped carbons for enhanced thermal conductivity and energy storage capacity. Appl Energy 2018;211:1203–15. https://doi.org/10.1016/j.apenergy.2017.12.025
Carly F, Vandermies M, Telek S, et al. Enhancing erythritol productivity in Yarrowia lipolytica using metabolic engineering. Metab Eng 2017;42:19–24. https://doi.org/10.1016/j.ymben.2017.05.002
Coccia G, Aquilanti A, Tomassetti S, Comodi G, Di Nicola G. Design, realization, and tests of a portable solar box cooker coupled with an erythritol-based PCM thermal energy storage. Sol Energy 2020;201:530–40. https://doi.org/10.1016/j.solener.2020.03.031
Anish R, Mariappan V, Joybari MM, Abdulateef AM. Performance comparison of the thermal behavior of xylitol and erythritol in a double spiral coil latent heat storage system. Therm Sci Eng Prog 2020;15:100441. https://doi.org/10.1016/j.tsep.2019.100441
Yuan M, Ye F, Xu C. Supercooling study of erythritol/EG composite phase change materials. Energy Procedia 2019;158:4629–34. https://doi.org/10.1016/j.egypro.2019.01.744
Junior JFR, Oliveski RDC, Rocha LAO, Biserni C. Numerical investigation on phase change materials (PCM): The melting process of erythritol in spheres under different thermal conditions. Int J Mech Sci 2018;148:20–30. https://doi.org/10.1016/j.ijmecsci.2018.08.006
Nazzi Ehms JH, De Césaro Oliveski R, Oliveira Rocha LA, Biserni C. Theoretical and numerical analysis on phase change materials (PCM): A case study of the solidification process of erythritol in spheres. Int J Heat Mass Transf 2018;119:523–32. https://doi.org/10.1016/j.ijheatmasstransfer.2017.11.124
Rakicka-Pustułka M, Mirończuk AM, Celińska E, Białas W, Rymowicz W. Scale-up of the erythritol production technology – Process simulation and techno-economic analysis. J Clean Prod 2020;257:120533. https://doi.org/10.1016/j.jclepro.2020.120533
Fleischer AS. Thermal energy storage using phase change materials: Fundamentals and applications. Springer, Cham, 2015. https://doi.org/10.1007/978-3-319-20922-7
Casini M. Phase-change materials. Smart Build. 2016;179–218. https://doi.org/10.1016/B978-0-08-100635-1.00005-8
Kuznik F, Johannes K, David D. Integrating phase change materials (PCMs) in thermal energy storage systems for buildings. Adv. Therm. Energy Storage Syst. Methods Appl. Woodhead Publishing Limited; 2014;325–56. https://doi.org/10.1533/9781782420965.2.325
Yuan M, Ren Y, Xu C, Ye F, Du X. Characterization and stability study of a form-stable erythritol/expanded graphite composite phase change material for thermal energy storage. Renew Energy 2019;136:211–22. https://doi.org/10.1016/j.renene.2018.12.107
Shen S, Tan S, Wu S, et al. The effects of modified carbon nanotubes on the thermal properties of erythritol as phase change materials. Energy Convers Manag 2018;157:41–8. https://doi.org/10.1016/j.enconman.2017.11.072
Wang Y, Wang L, Xie N, Lin X, Chen H. Experimental study on the melting and solidification behavior of erythritol in a vertical shell-and-tube latent heat thermal storage unit. Int J Heat Mass Transf 2016;99:770–81. https://doi.org/10.1016/j.ijheatmasstransfer.2016.03.125
Singh R, Sadeghi S, Shabani B. Thermal conductivity enhancement of phase change materials for low-temperature thermal energy storage applications. Energies 2019;12(1). https://doi.org/10.3390/en12010075
Sheng N, Dong K, Zhu C, Akiyama T, Nomura T. Thermal conductivity enhancement of erythritol phase change material with percolated aluminum filler. Mater Chem Phys 2019;229:87–91. https://doi.org/10.1016/j.matchemphys.2019.02.033
Leng G, Qiao G, Xu G, Vidal T, Ding Y. Erythritol-Vermiculite form-stable phase change materials for thermal energy storage. Energy Procedia 2017;142:3363–8. https://doi.org/10.1016/j.egypro.2017.12.471
Oya T, Nomura T, Tsubota M, Okinaka N, Akiyama T. Thermal conductivity enhancement of erythritol as PCM by using graphite and nickel particles. Appl Therm Eng 2013;61(2):825–8. https://doi.org/10.1016/j.applthermaleng.2012.05.033
Soni V, Kumar A, Jain VK. Performance evaluation of nano-enhanced phase change materials during discharge stage in waste heat recovery. Renew Energy 2018;127:587–601. https://doi.org/10.1016/j.renene.2018.05.009
Qu L. Investigating the Relationship between Salinity and Specific Heat Capacity. Queensl Acad n.d. http://nexusstem.co.uk/wp-content/uploads/2017/01/Queensland-Academies-1.pdf
Knowino. Density (chemistry), https://www.tau.ac.il/~tsirel/dump/Static/knowino.org/wiki/Density_(chemistry).html
Brown HM. The thermal conductivity of sodium chloride at elevated temperatures. PhD Thesis, University of Missouri, 1955. https://scholarsmine.mst.edu/cgi/viewcontent.cgi?article=3590&context=masters_theses
Ferguson JB. The melting and freezing point of Sodium Chloride. J Phys Chem 1921;26(7):626–30
Zeng JL, Chen YH, Shu L, et al. Preparation and thermal properties of exfoliated graphite/erythritol/mannitol eutectic composite as form-stable phase change material for thermal energy storage. Sol Energy Mater Sol Cells 2018;178:84–90. https://doi.org/10.1016/j.solmat.2018.01.012
Shin HK, Rhee KY, Park SJ. Effects of exfoliated graphite on the thermal properties of erythritol-based composites used as phase-change materials. Compos Part B Eng 2016;96:350–3. https://doi.org/10.1016/j.compositesb.2016.04.033
Vivekananthan M, Amirtham VA. Characterisation and thermophysical properties of graphene nanoparticles dispersed erythritol PCM for medium temperature thermal energy storage applications. Thermochim Acta 2019;676:94–103. https://doi.org/10.1016/j.tca.2019.03.037
Shawe J, Riesen R, Widmann J, Schubnell M. Interpreting DSC curves Part 1: Dynamic measurements. 2000. https://www.eng.uc.edu/~beaucag/Classes/Characterization/DSCParts/Artifacts%20in%20DSC%20Usercom_11.pdf
Calculate Activation Energy from TGA data using Origin Software. Nanoencryption https://youtu.be/eLqShUApVXM?t=403
Moon B, Jun N, Park S, Seok CS, Hong US. A study on the modified Arrhenius equation using the oxygen permeation block model of crosslink structure. Polymers (Basel) 2019;11(1). https://doi.org/10.3390/polym11010136
Peleg M, Normand MD, Corradini MG. The Arrhenius equation revisited. Crit Rev Food Sci Nutr 2012;52(9):830–51. https://doi.org/10.1080/10408398.2012.667460
Laidler KJ. The development of the arrhenius equation. J Chem Educ 1984;61(6):494–8. https://doi.org/10.1021/ed061p494
Camirand CP. Measurement of thermal conductivity by differential scanning calorimetry. Thermochim Acta 2004;417(1):1–4. https://doi.org/10.1016/j.tca.2003.12.023
Camirand C. Étude de la chaleur spécifique et de la conductivité thermique des hydrures métalliques par calorimétrie différentielle. Thèse et mémoire, Université du Québec à Trois-Rivières, 2000. https://depot-e.uqtr.ca/id/eprint/3139 in French
Flynn JH, Levin DM. A method for the determination of thermal conductivity of sheet materials by differential scanning calorimetry (DSC). Thermochim Acta 1988;126:93–100. https://doi.org/10.1016/0040-6031(88)87254-X
Niezgoda-Zelasko B. The Enthalpy-porosity Method Applied to the Modelling of the Ice Slurry Melting Process during Tube Flow. Procedia Eng 2016;157:114–21. https://doi.org/10.1016/j.proeng.2016.08.346
Eanest Jebasingh B, Valan Arasu A. A comprehensive review on latent heat and thermal conductivity of nanoparticle dispersed phase change material for low-temperature applications. Energy Storage Mater 2020;24:52–74. https://doi.org/10.1016/j.ensm.2019.07.031
Okoro HK, Odebunmi EO. Kinetics and mechanism of oxidation of sugar and sugar alcohols by KMnO4. Int J Phys Sci 2009;4(9): 673ED0819348. https://doi.org/10.5897/IJPS.9000340
Mayilvelnathan V, Valan Arasu A. Experimental investigation on thermal behavior of graphene dispersed erythritol PCM in a shell and helical tube latent energy storage system. Int J Therm Sci 2020;155:106446. https://doi.org/10.1016/j.ijthermalsci.2020.106446
Gunasekara SN, Stalin J, Marçal M, et al. Erythritol, glycerol, their blends, and olive oil, as sustainable phase change materials. Energy Procedia 2017;135:249–62. https://doi.org/10.1016/j.egypro.2017.09.517
MINITAB. Interpolation method for mesh. MINITAB. https://support.minitab.com/en-us/minitab/18/help-and-how-to/graphs/how-to/general-graph-options/display-options/interpolation-method-for-mesh/