Kinetic behaviour of the roasting/selective reduction process with the use of a mixture of bituminous coal and fuel oil as the additive Technical paper
Main Article Content
Abstract
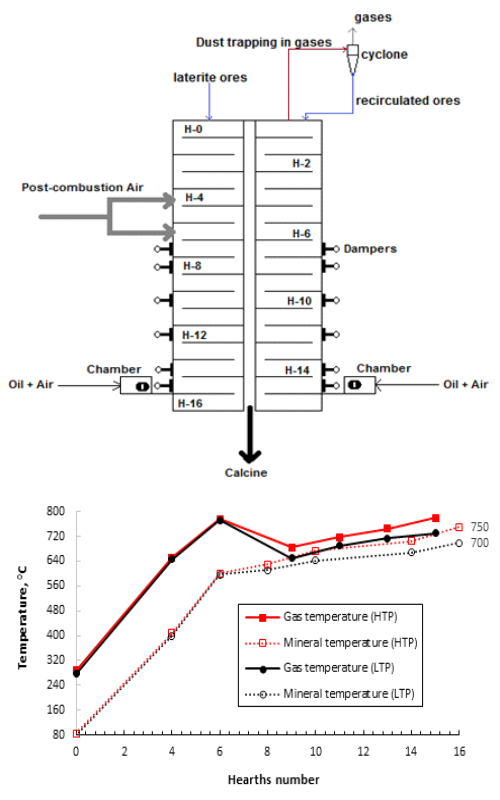
Lateritic ore is currently the main raw material that allows extraction of Ni by the Caron process. To date, the kinetic behaviour of the roasting/selective reduction process of lateritic nickeli¬ferous ores on a pilot scale is largely unknown. In the present study, the kinetic behaviour, the controlling stage and the mechanisms that describe this process with the use of a mixture of 2 wt.% bituminous coal and 1.25 wt.% fuel oil as a reducing additive were determined during the evaluation of the high and low thermal profiles, respectively. The phases of the reduced/leached minerals and the fed ore were analysed by X-ray powder diffraction. It was observed that the mixture used as a reducing additive guarantees an adequate trans¬for¬mation in both thermal profiles; the relationship between the residual Ni and residence time is described by a first-order reaction with the determination coefficients greater than 0.949. Although the influence of post-combustion air is not analysed, the controlling stage was diffusion through the ash layer with an activation energy of 14.4060 kJ mol-1. Thus, the most precise combination to describe the process is diffusion through the ash layer and the growth of the nuclei.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
References
[1] Bartzas G, Tsakiridis PE, Komnitsas K. Nickel industry: Heavy metal(loid)s contamination - sources, environmental impacts and recent advances on waste valorization. Curr Opin Environ Sci Heal. 2021; 21: 100253. https://doi.org/10.1016/j.coesh.2021.100253
[2] Mitterecker J, Košević M, Stopic S, Friedrich B, Panić V, Stevanović J, Mihailović M. Electrochemical Investigation of Lateritic Ore Leaching Solutions for Ni and Co Ions Extraction. Metals (Basel). 2022; 12(2): 325 https://doi.org/10.3390/met12020325
[3] Hu X, Ma B, He F, Chen Y, Wang C. Mineralogical characterization of low-grade nickel laterites from the North Oman Mountains: Using mineral liberation analyses - scanning electron microscopy-based automated quantitative mineralogy. Ore Geol Rev. 2020; 120: 103429. https://doi.org/10.1016/j.oregeorev.2020.103429
[4] Zevgolis EN, Daskalakis KA. The Nickel Production Methods from Laterites and the Greek Ferronickel Production among Them. Mater Proc. 2021; 5(1): 104. https://doi.org/10.3390/materproc2021005104.
[5] Pintowantoro S, Widyartha AB, Setiyorini Y, Abdul F. Sodium Thiosulfate and Natural Sulfur: Novel Potential Additives for Selective Reduction of Limonitic Laterite Ore. J Sustain Metall .2021; 7(2): 481-494. doi: 10.1007/s40831-021-00352-4.
[6] Moats MS, Davenport WG: Chapter 2.3 Nickel and Cobalt, In Treatise on Process Metallurgy. 2nd ed. Elsevier; 2024: 575-604. https://doi.org/10.1016/B978-0-323-85373-6.00030-2.
[7] Caron MH. Fundamental and practical factors in ammonia leaching of nickel and cobalt ores. JOM. 1950; 2(1): 67-90. https://doi.org/10.1007/BF03398981.
[8] Caron MH. “Separation of Nickel and Cobalt” JOM. 1950; 2(1): 91-103. https://doi.org/10.1007/BF03398982.
[9] Kießling F, Stopic S, Gürmen S, Friedrich B. Recovery of Diamond and Cobalt Powders from Polycrystalline Drawing Die Blanks via Ultrasound Assisted Leaching Process—Part 2: Kinetics and Mechanisms. Metals (Basel) 2020; 10(6): 741. https://doi.org/10.3390/met10060741.
[10] Rodriguez R. Reduction in energy cost in Cuban Caron Process Plants. In: International Laterite Nickel Symposium 2004 (as held during the 2004 TMS Annual Meeting). The Minerals, Metals & Materials Society 2004, pp. 657-664. ISBN: 0-87339-550-6.
[11] Caetano GC, Ostroski IC, de Barros MASD. Lateritic Nickel and Cobalt Recovery Routes: Strategic Technologies. Miner Process Extr Metall Rev. 2024; 400-414. https://doi.org/10.1080/08827508.2024.2328696.
[12] Canterford Jh. Oxide Ores of Nickel — The Australian Situation. Miner Process Extr Metall Rev. 1983; 1(1-2): 85-109. https://doi.org/10.1080/08827508308952590.
[13] Coello-Velázquez AL, Quijano Arteaga V, Menéndez-Aguado JM, Pole FM, Llorente L. Use of the Swebrec Function to Model Particle Size Distribution in an Industrial-Scale Ni-Co Ore Grinding Circuit. Metals (Basel). 2019; 9(8): 882. https://doi.org/10.3390/met9080882.
[14] De Graaf JE. The treatment of lateritic nickel ores — a further study of the caron process and other possible improvements. Part I. Effect of reduction conditions. Hydrometallurgy 1979; 5(1): 47-65. https://doi.org/10.1016/0304-386X(79)90027-6.
[15] Mano ES., Caner L., Petit S., Chaves AP., Mexias, A. S. Ni-smectitic ore behaviour during the Caron process. Hydrometallurgy. 2019; 186: 200-209. https://doi.org/10.1016/j.hydromet.2019.04.010.
[16] Angulo Palma HJ, Legrá AL, Urgellés AL, Gálvez E, Castillo J. Post-combustion Effect on Nickel and Cobalt Extractions from the Caron Process. In: Bindhu V, Tavares JM, Ţălu Ş, eds. Proceedings of Fourth International Conference on Inventive Material Science Applications. Springer, Singapore 2022; 515-527. https://doi.org/10.1007/978-981-16-4321-7_43.
[17] Ramírez Pérez IM, Ramírez Serrano B. Efecto de la postcombustión sobre los principales índices técnico-económicos en un horno Herreshoff para la producción de níquel. Post-combustion effect on the main technical-economic indices in the Herreshoff furnace for nickel production. Min Geol. 2021; 37(4): 426-444. (Spanish).
[18] Angulo Palma HJ, Terencio Guevara PL, Legrá AL, Videaux Arcia L. Análisis especiales en un horno de reducción de níquel a escala de Planta Piloto. Special Analysis in a Nickel Reduction Furnace at Pilot Plant scale. RTQ. 2017; 37(3): 484-499. (Spanish).
[19] Pickles CA, Anthony W. A Thermodynamic Study of the Reduction of a Limonitic Laterite Ore by Methane. High Temp Mater Proc. 2018; 37(9-10): 909-919. https://doi.org/10.1515/htmp-2017-0106.
[20] Pickles CA, Anthony W. Thermodynamic modelling of the reduction of a saprolitic laterite ore by methane. Miner Eng. 2018; 120: 47-59. https://doi.org/10.1016/j.mineng.2018.02.006.
[21] Ilyas S, Kim H, Srivastava RR. Carbothermic Reduction Roasting of a Low-Grade Nickel Laterite Ore in the Modified Caron Process. In: C. Anderson et al., eds. The 5th International Symposium on Nickel and Cobalt. Switzerland: Springer Nature; 2021: 317-328. https://doi.org/10.1007/978-3-030-65647-8_27.
[22] Ilyas S, Srivastava RR, Kim H, Ilyas N, Sattar R. Extraction of nickel and cobalt from a laterite ore using the carbothermic reduction roasting-ammoniacal leaching process. Sep Purif Technol. 2020; 232: 115971. https://doi.org/10.1016/j.seppur.2019.115971.
[23] Angulo Palma HJ, Legrá AL, Hernández Pedrera C, Lamorú Urgellés A, Vega Cala RJ. Efecto de la sustitución del petróleo aditivo por carbón bituminoso en el proceso de reducción de lateritas. Effect Substit Addit Oil with Bitum Coal Process Reducing Laterites. RTQ. 2018; 38: 750-764. (Spanish).
[24] De Alvarenga Oliveira V, dos Santos CG, de Albuquerque Brocchi E. Assessing the Influence of NaCl on the Reduction of a Siliceous Laterite Nickel Ore Under Caron Process Conditions. Metall Mater Trans B. 2019; 50(3): 1309-1321. https://doi.org/https://doi.org/10.1007/s11663-019-01552-w.
[25] Angulo Palma HJ, Legrá AL, Hernández Pdrera C, Lamorú Urgellés A, Toro Villarroel N. Reducción de menas lateríticas utilizando como aditivo mezclas de carbón bituminoso y petróleo. Reduction of lateritic minerals using additive mixtures of bituminous coal and oil. RTQ. 2020; 40: 93-105. (Spanish).
[26] Angulo Palma HJ., Legrá AL., Lamorú Urgellés A., Hernández Pedrera C., Gallegos S., Galleguillos Madrid FM., Toro Villarroel, N. Use of a mixture of coal and oil as an additive for selective reduction of lateritic ore by the Caron process: Hem Ind. 2024; 78: 17-27. https://doi.org/10.2298/HEMIND230118017A.
[27] Sant B. Chemical reaction engineering. Talanta. 1968; 15(12): 1483-1486. https://doi.org/10.1016/0039-9140(68)80211-5.
[28] Levenspiel O. Chemical Reaction Engineering. 3rd ed., New York, NY: John Wiley & Sons; 1999. ISBN 0-471-25424-X.
[29] Li B., Ding Z., Wei Y., Wang H., Yang Y., Barati M. Kinetics of reduction of low-grade nickel laterite ore using carbon monoxide. Metall Mater Trans B. 2018; 49: 3067-3073. https://doi.org/10.1007/s11663-018-1367-8.
[30] Cabrera G, Gómez JM, Hernández I, Coto O, Cantero D. Different strategies for recovering metals from CARON process residue. J Hazard Mater. 2011; 189(3): 836-42. https://doi.org/10.1016/j.jhazmat.2011.03.048.
[31] Rhamdhani MA, Chen J., Hidayat T, Jak E., Hayes P. Advances in research on nickel production through the Caron process. In: Harre J, ed. Proceedings of European Metallurgical Conference 2009. Germany: GDMB; 2009: 899-914. ISBN 978-3-940276-19-3.
[32] Rhamdhani MA, Hayes PC, Jak E. Nickel laterite Part 1-microstructure and phase characterisations during reduction roasting and leaching. Miner Process Extr. 2009; 118: 129-145. https://doi.org/10.1179/174328509X431391.
[33] Rhamdhani MA, Hayes PC, Jak E. Nickel laterite Part 2-thermodynamic analysis of phase transformations occurring during reduction roasting. Miner Process Extr. 2009; 118: 146-155. https://doi.org/10.1179/174328509X431409.
[34] Valix M, Cheung W. Effect of sulfur on the mineral phases of laterite ores at high temperature reduction. Miner Eng. 2002; 15(7): 523-530. https://doi.org/10.1016/S0892-6875(02)00069-9.
[35] Valix M, Cheung WH. Study of phase transformation of laterite ores at high temperature. Miner Eng. 2002; 15(8): 607-612. https://doi.org/10.1016/S0892-6875(02)00068-7.
[36] Shoubao L, Eng B, Eng M. Study of nickeliferrous laterite reduction. A Thesis Submitted to the School of Graduate Studies in Partial Fulfilment of the Requirements for the Degree of Master Engineering, University of Science and Technology, Beijing; 1999.https://translate.google.com/?sl=en&tl=es&text=Study%20of%20nickeliferrous%20laterite%20reduction&op=translate.
[37] Castellanos Suárez J. Cinética de la reducción de los minerales oxidados de níquel en Cuba. Kinetics of the reduction of oxidized nickel minerals in Cuba. Min Geol. 1984; 2: 197-222. (Spanish).
[38] Shofi A, Supriyatna Y, Prasetyo AB. Selective Reduction of Southeast Sulawesi Nickel Laterite using Palm Kernel Shell Charcoal: Kinetic Studies with Addition of Na2SO4 and NaCl as Additives. Bull Chem React Eng Catal. 2020; 15(2), 501-513. https://doi.org/10.9767/bcrec.15.2.7733.501-513.