Valorization of soybean meal to produce high-protein animal feed and value-added products using a new strain of Aureobasidium pullulans Original scientific paper
Main Article Content
Abstract
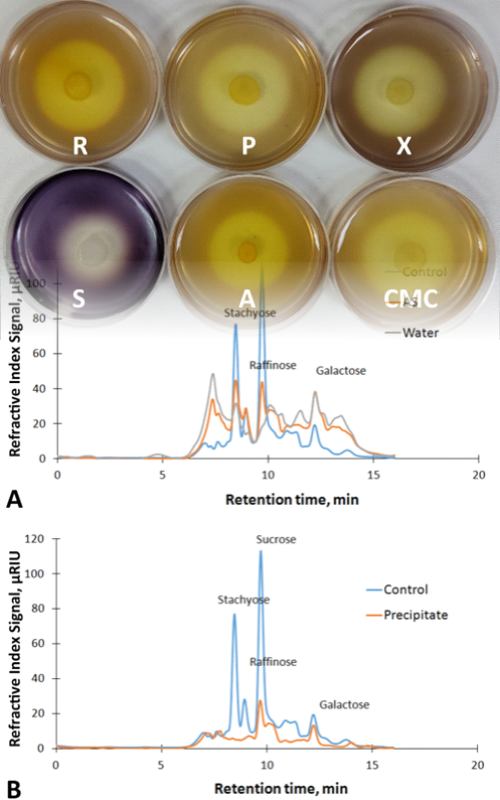
Soybean meal (SBM) is a by-product of soybean oil production. It is a high-quality protein supplement for animal feed. However, it is rich in anti-nutritive factors and indigestible components, among which the special attention is focused on galacto-oligosaccharides, due to the lack of α-galactosidase in monogastric animals. The main goal of this study was to apply fermentation using a selected strain of black yeast-like fungus of the SBM to obtain a high-protein, low-oligosaccharide soy-based product. Screening for an appropriate strain of Aureobasidium spp. among natural isolates from grapes has been performed. The highest α-galactosidase activity of 0.89 U cm-3 was produced by the strain identified as A. pullulans P8. It was applied in SBM submerged (SmF) and solid-state fermentations (SSF). Maximal crude protein yield (~61 % based on dry weight) and the lowest galacto-oligosaccharides content were obtained after 3 days of SmF at 30 °C and 10 % of dry matter. SSF produced ~58 % crude protein after 7 days of incubation at 30 °C with substrate containing 30 % of dry matter. Extracellular enzymatic activities of cellulase, pectinase, amylase, xylanase, and α-galactosidase were detected in the supernatant after SmF, indicating its potential for hydrolysis of various lignocellulosic biomass substrates.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-65/2024-03/200135;451-03-66/2024-03/200287 -
Innovation Fund of the Republic of Serbia
Grant numbers Voucher number 1076
References
[1] ReportLinker. https://www.reportlinker.com/clp/global/3685. Accessed June 26, 2025.
[2] Shiu YL, Wong SL, Guei WC, Shin YC, Liu CH. Increase in the plant protein ratio in the diet of white shrimp, Litopenaeus vannamei (Boone), using Bacillus subtilis E20-fermented soybean meal as a replacement. Aquac Res. 2015; 46(2): 382-394. https://doi.org/10.1111/are.12186
[3] Karr-Lilienthal LK, Kadzere CT, Grieshop CM, Fahey GC. Chemical and nutritional properties of soybean carbohydrates as related to nonruminants. Livest Prod Sci. 2005; 97(1): 1-12. https://doi.org/10.1016/j.livprodsci.2005.01.015
[4] Abdel-Raheem SM, Mohammed ESY, Mahmoud RE, Gamal MFE, Nada HS, El-Ghareeb WR, Marzok M, Meligy AMA, Abdulmohsen M, Ismail H, Ibrahim D, Kishawy ATY. Double-Fermented Soybean Meal Totally Replaces Soybean Meal in Broiler Rations with Favorable Impact on Performance, Digestibility, Amino Acids Transporters and Meat Nutritional Value. Animals. 2023; 13(6): 1030. https://doi.org/10.3390/ani13061030
[5] Middelbos IS, Fahey GC. Soybean Carbohydrates. In: Johnson LA, White PJ, Galloway R, eds. Soybeans Chemistry, Production, Processing, and Utilization. Urbana, IL: AOCS Press; 2008. p. 269-296. https://doi.org/10.1016/B978-1-893997-64-6.50012-3
[6] Anisha GS. Microbial α-galactosidases: Efficient biocatalysts for bioprocess technology. Bioresour Technol 2022; 344: 126293. https://doi.org/10.1016/j.biortech.2021.126293
[7] Celi P, Cowieson AJ, Fru-Nji F, Steinert RE, Kluenter A-M, Verlhac V. Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal production. Anim Feed Sci Technol. 2017; 234: 88-100. https://doi.org/10.1016/j.anifeedsci.2017.09.012
[8] Zhu J, Gao M, Zhang R, et al. Effects of soybean meal fermented by L. plantarum, B. subtilis and S. cerevisieae on growth, immune function and intestinal morphology in weaned piglets. Microb Cell Fact. 2017; 16(1): 191. https://doi.org/10.1186/s12934-017-0809-3
[9] Shang QH, Ma XK, Li M, Zhang LH, Hu JX, Piao XS. Effects of α-galactosidase supplementation on nutrient digestibility, growth performance, intestinal morphology and digestive enzyme activities in weaned piglets. Anim Feed Sci Technol. 2018; 236: 48-56. https://doi.org/10.1016/j.anifeedsci.2017.11.008
[10] Milić MD, Buntić A V., Mihajlovski KR, Ilić N V., Davidović SZ, Dimitrijević-Branković SI. The development of a combined enzymatic and microbial fermentation as a viable technology for the spent coffee ground full utilization. Biomass Convers Biorefinery. 2023; 13(8): 6747-6759. https://doi.org/10.1007/s13399-021-01605-8
[11] Jakobsen G V., Jensen BB, Bach Knudsen KE, Canibe N. Impact of fermentation and addition of non-starch polysaccharide-degrading enzymes on microbial population and on digestibility of dried distillers grains with solubles in pigs. Livest Sci. 2015; 178: 216-227. https://doi.org/10.1016/j.livsci.2015.05.028
[12] Senanayake D, Torley PJ, Chandrapala J, Terefe NS. Microbial Fermentation for Improving the Sensory, Nutritional and Functional Attributes of Legumes. Fermentation. 2023;.9(7): 635. https://doi.org/10.3390/fermentation9070635
[13] Tang J, Li W, Zhou Q, et al. Effect of heating, microbial fermentation, and enzymatic hydrolysis of soybean meal on growth performance, nutrient digestibility, and intestinal microbiota of weaned piglets. J Anim Sci. 2023; 101: skad384. https://doi.org/10.1093/jas/skad384
[14] Xue J, Wu J, Ji Y, et al. Effect of microbial fermentation on the quality of soybean meal. Int J Food Sci Technol. 2024; 59(1): 72-83. https://doi.org/10.1111/ijfs.16817
[15] Baker KM, Liu Y, Stein HH. Nutritional value of soybean meal produced from high protein, low oligosaccharide, or conventional varieties of soybeans and fed to weanling pigs. Anim Feed Sci Technol. 2014; 188: 64-73. https://doi.org/10.1016/j.anifeedsci.2013.10.018
[16] Loman A Al, Ju LK. Soybean carbohydrate as fermentation feedstock for production of biofuels and value-added chemicals. Process Biochem. 2016; 51(8): 1046-1057. https://doi.org/10.1016/j.procbio.2016.04.011
[17] Deng Z, Duarte ME, Kim SY, Hwang Y, Kim SW. Comparative effects of soy protein concentrate, enzyme-treated soybean meal, and fermented soybean meal replacing animal protein supplements in feeds on growth performance and intestinal health of nursery pigs. J Anim Sci Biotechnol. 2023; 14(1): 89. https://doi.org/10.1186/s40104-023-00888-3
[18] Chen S, Zheng H, Gao J, Song H, Bai W. High-level production of pullulan and its biosynthesis regulation in Aureobasidium pullulans BL06. Front Bioeng Biotechnol. 2023; 11. https://doi.org/10.3389/fbioe.2023.1131875
[19] Cruz-Santos MM, Antunes FAF, Arruda GL, Shibukawa VP, Prado, CA, Ortiz-Silos N, Castro-Alonso MJ, Marcelino PRF, Santos JC. Production and applications of pullulan from lignocellulosic biomass: Challenges and perspectives. Bioresour Technol. 2023; 385: 129460. https://doi.org/10.1016/j.biortech.2023.129460
[20] Thirumavalavan K, Manikkadan TR, Dhanasekar R. Pullulan production from coconut by-products by Aureobasidium pullulans. African J Biotechnol. 2009; 8(2): 254-258
[21] Wang P, Jia S-L, Liu G-L, Chi Z, Chi Z-M. Aureobasidium spp. and their applications in biotechnology. Process Biochem. 2022; 116: 72-83. https://doi.org/10.1016/j.procbio.2022.03.006
[22] Di Francesco A, Sciubba L, Bencivenni M, Marzadori C, Baraldi E. Application of Aureobasidium pullulans in iron‐poor soil. Can the production of siderophores improve iron bioavailability and yeast antagonistic activity? Ann Appl Biol. 2022; 180(3): 398-406. https://doi.org/10.1111/aab.12742
[23] Rensink S, van Nieuwenhuijzen EJ, Sailer MF, Struck C, Wösten HAB. Use of Aureobasidium in a sustainable economy. Appl Microbiol Biotechnol. 2024; 108(1): 202. https://doi.org/10.1007/s00253-024-13025-5
[24] Carević M, Banjanac K, Ćorović M, et al. Selection of lactic acid bacteria strain for simultaneous production of α- and β-galactosidases. Zast Mater. 2016; 57(2): 265-273. https://doi.org/10.5937/zasmat1602265c
[25] Miller GL. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal Chem. 1959; 31(3): 426-428. https://doi.org/10.1021/ac60147a030
[26] Ilić N, Davidović S, Milić M, et al. Valorization of lignocellulosic wastes for extracellular enzyme production by novel Basidiomycetes: screening, hydrolysis, and bioethanol production. Biomass Convers Biorefinery. 2022; 13: 17175-17186. https://doi.org/10.1007/s13399-021-02145-x
[27] ISO 5983-1:2005; Animal feeding stuffs: Determination of nitrogen content and calculation of crude protein content. Part 1: Kjeldahl method, International Standard Organization. Technical Committee: Geneva, Switzerland, 2005; ISO/TC 34/SC 10. https://www.iso.org/standard/39145.html
[28] Thermo Fisher Scientific. Chromeleon (Version 7.2) [Computer Software]. Thermo Fisher Scientific, 2018. https://www.thermofisher.com/order/catalog/product/CHROMELEON7
[29] OriginLab Corporation. OriginPro (Version 9.0) [Computer Software]. OriginLab Corporation, 2012. https://www.originlab.com/
[30] van Nieuwenhuijzen EJ. Aureobasidium. Encycl Food Microbiol Second Ed 2014; 1: 105-109. https://doi.org/10.1016/B978-0-12-384730-0.00017-3
[31] Bankeeree W, Lotrakul P, Prasongsuk S, Kim SW, Punnapayak H. Enhanced Production of Cellulase-Free Thermoactive Xylanase Using Corncob by a Black Yeast, Aureobasidium pullulans CBS 135684. Korean Chem Eng Res. 2016; 54(6): 822-829. https://doi.org/10.9713/kcer.2016.54.6.822
[32] Mulay YR, Deopurkar RL. Production of amylase from indigenously isolated strain of Aureobasidium Pullulans and its hyper producing mutant. J Microbiol Biotechnol Food Sci. 2017; 7(3): 287-293. https://doi.org/10.15414/jmbfs.2017/18.7.3.287-293
[33] Zajc J, Černoša A, Francesco A Di, et al. Fungal Genomics & Biology Characterization of Aureobasidium pullulans Isolates Selected as Biocontrol Agents Against Fruit Decay Pathogens. Fungal Genom Biol. 2020; 10(1): 163. https://doi.org/10.35248/2165-8056.20.10.163
[34] Leite RSR, Bocchini DA, Martins EDS, Silva D, Gomes E, Da Silva R. Production of cellulolytic and hemicellulolytic enzymes from Aureobasidium pulluans on solid state fermentation. Appl Biochem Biotechnol. 2007; 137-140(1-12): 281-288. https://doi.org/10.1007/s12010-007-9058-y
[35] Thakur M, Hurburgh CR. Quality of US soybean meal compared to the quality of soybean meal from other origins. JAOCS, J Am Oil Chem Soc. 2007; 84(9): 835-843. https://doi.org/10.1007/s11746-007-1107-8
[36] Badr-Eldin SM, El-Tayeb OM, El-Masry HG, Mohamad FHA, El-Rahman OAA. Polysaccharide production by Aureobasidium pullulans: factors affecting polysaccharide formation. World J Microbiol Biotechnol. 1994; 10(4): 423-426. https://doi.org/10.1007/BF00144465
[37] Lotrakul P, Unhapattaratitikul P, Seelanan T, Prasongsuk S, Punnapayak H. An aubasidan-like β-glucan produced by Aureobasidium pullulans in Thailand. ScienceAsia. 2013; 39(4): 363. https://doi.org/10.2306/scienceasia1513-1874.2013.39.363
[38] Zhang Y, Ishikawa M, Koshio S, et al. Optimization of soybean meal fermentation for aqua-feed with bacillus subtilis natto using the response surface methodology. Fermentation. 2021; 7(4): 306. https://doi.org/10.3390/fermentation7040306
[39] Ilić N, Milić M, Beluhan S, Dimitrijević-Branković S. Cellulases: From Lignocellulosic Biomass to Improved Production. Energies. 2023; 16(8): 3598. https://doi.org/10.3390/en16083598
[40] Miljkovic M, Davidovic S, Djukic-Vukovic A, et al. Utilization of agro-industrial by-products as substrates for dextransucrase production by Leuconostoc mesenteroides T3: Process optimization using response surface methodology. Hem Ind. 2021; 75(3): 135-146. https://doi.org/10.2298/HEMIND200710015M
[41] Mihajlovski K, Davidovic S, Veljovic D, Carevic M, Lazic V, Dimitrijevic-Brankovic S. Effective valorization of barley bran for simultaneous cellulase and β-amylase production by Paenibacillus chitinolyticus CKS1: Statistical optimization and enzymes application. J Serbian Chem Soc. 2017; 82(11): 1223-1236. https://doi.org/10.2298/JSC170514092M
[42] Salim AA, Grbavčić S, Šekuljica N, et al. Enzyme production by solid‐state fermentation on soybean meal: A comparative study of conventional and ultrasound‐assisted extraction methods. Biotechnol Appl Biochem. 2019; 66(3): 361-368. https://doi.org/10.1002/bab.1732
[43] Ademakinwa AN, Agboola FK. Kinetic and thermodynamic investigations of cell-wall degrading enzymes produced by Aureobasidium pullulans via induction with orange peels: application in lycopene extraction. Prep Biochem Biotechnol. 2019; 49(10): 949-60. https://doi.org/10.1080/10826068.2019.1650375
[44] Gostinčar C, Ohm RA, Kogej T, et al. Genome sequencing of four Aureobasidium pullulans varieties: Biotechnological potential, stress tolerance, and description of new species. BMC Genomics. 2014;.15(1): 549 . https://doi.org/10.1186/1471-2164-15-549
[45] Yegin S, Buyukkileci AO, Sargin S, Goksungur Y. Exploitation of Agricultural Wastes and By-Products for Production of Aureobasidium pullulans Y-2311-1 Xylanase: Screening, Bioprocess Optimization and Scale Up. Waste and Biomass Valorization. 2017; 8(3): 999-1010. https://doi.org/10.1007/s12649-016-9646-6
[46] Bennamoun L, Hiligsmann S, Dakhmouche S, et al. Production and Properties of a Thermostable, pH—Stable Exo-Polygalacturonase Using Aureobasidium pullulans Isolated from Saharan Soil of Algeria Grown on Tomato Pomace. Foods. 2016; 5(4): 72. https://doi.org/10.3390/foods5040072
[47] Alhomodi AF, Gibbons WR, Karki B. Estimation of cellulase production by Aureobasidium pullulans, Neurospora crassa, and Trichoderma reesei during solid and submerged state fermentation of raw and processed canola meal. Bioresour Technol Reports. 2022; 18: 101063. https://doi.org/10.1016/j.biteb.2022.101063
[48] Vieira MM, Kadoguchi E, Segato F, da Silva SS, Chandel AK. Production of cellulases by Aureobasidium pullulans LB83: optimization, characterization, and hydrolytic potential for the production of cellulosic sugars. Prep Biochem Biotechnol 2021; 51(2): 153-163. https://doi.org/10.1080/10826068.2020.1799393
[49] Qian Y, Zhong L, Sun Y, et al. Enhancement of Cellulase Production in Trichoderma reesei via Disruption of Multiple Protease Genes Identified by Comparative Secretomics. Front Microbiol. 2019; 10. https://doi.org/10.3389/fmicb.2019.02784