Sprovođenje hitnih mera za poboljšanje efikasnosti uklanjanja nikla iz vode na postojećem postrojenju za prečišćavanje vode Naučni rad
Glavni sadržaj članka
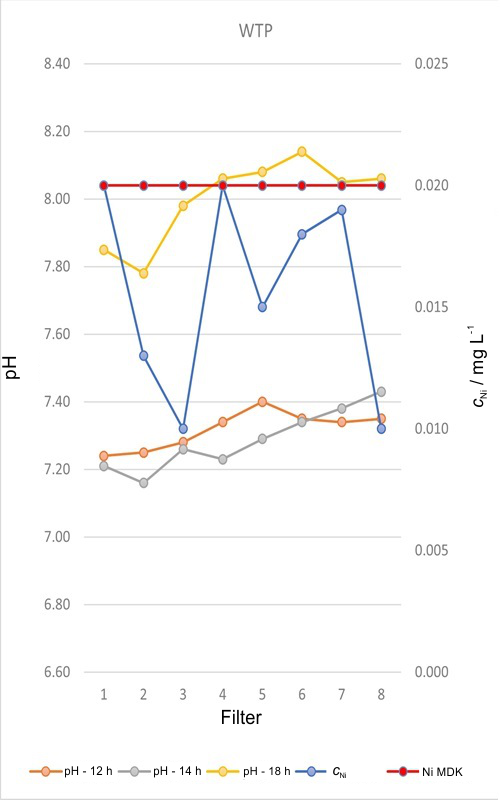
Apstrakt
Detalji članka
Broj časopisa
Rubrika

Ovaj rad je pod Creative Commons Autorstvo 4.0 Internacionalna licenca.
Kada je rukopis prihvaćen za objavlјivanje, autori prenose autorska prava na izdavača. U slučaju da rukopis ne bude prihvaćen za štampu u časopisu, autori zadržavaju sva prava.
Na izdavača se prenose sledeća prava na rukopis, uklјučujući i dodatne materijale, i sve delove, izvode ili elemente rukopisa:
- pravo da reprodukuje i distribuira rukopis u štampanom obliku, uklјučujući i štampanje na zahtev;
- pravo na štampanje probnih primeraka, reprint i specijalnih izdanja rukopisa;
- pravo da rukopis prevede na druge jezike;
- pravo da rukopis reprodukuje koristeći fotomehanička ili slična sredstva, uklјučujući, ali ne ograničavajući se na fotokopiranje, i pravo da distribuira ove kopije;
- pravo da rukopis reprodukuje i distribuira elektronski ili optički koristeći sve nosioce podataka ili medija za pohranjivanje, a naročito u mašinski čitlјivoj/digitalizovanoj formi na nosačima podataka kao što su hard disk, CD-ROM, DVD, Blu-ray Disc (BD), mini disk, trake sa podacima, i pravo da reprodukuje i distribuira rukopis sa tih prenosnika podataka;
- pravo da sačuva rukopis u bazama podataka, uklјučujući i onlajn baze podataka, kao i pravo prenosa rukopisa u svim tehničkim sistemima i režimima;
- pravo da rukopis učini dostupnim javnosti ili zatvorenim grupama korisnika na osnovu pojedinačnih zahteva za upotrebu na monitoru ili drugim čitačima (uklјučujući i čitače elektonskih knjiga), i u štampanoj formi za korisnike, bilo putem interneta, onlajn servisa, ili putem internih ili eksternih mreža.
Kako citirati
Reference
[1] Rulebook on the hygienic suitability of drinking water. The Official Gazette of the FRY 42/98; 44/99; and Official Gazette of RS 28/19 (Pravilnik o ispravnosti vode za piće. Službeni glasnik RS 42/98; 44/99; 28/19; in Serbian) https://www.paragraf.rs/propisi/pravilnik-higijenskoj-ispravnosti-vode-pice.html
[2] Kutir P, College PG, Chakkey, J. Effect of pH on the removal of Ni (II). Int J Chem Sci. 2013; 11(3): 1493-1497. https://www.tsijournals.com/articles/effect-of-ph-on-the-removal-of-ni-ii.pdf
[3] Cruz-Lopes LP, Morgana M, Bruno E, Raquel PFG, Ideal pH for the adsorption of metal ions Cr6+, Ni2+, Pb2+ in aqueous solution with different adsorbent materials. Open Agric. 2021; 6:115-123. https://doi.org/10.1515/opag-2021-0225
[4] Hala AH. Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC J. 2013; 9(3):276-282. https://doi.org/10.1016/j.hbrcj.2013.08.004
[5] Utkarsh U, Sreedhar I. Singh SA, Patel CM, Anitha KL, Recent advances in heavy metal removal by chitosan based adsorbents Carbohydr Polym. 2021; 251: 117000 . https://doi.org/10.1016/j.carbpol.2020.117000
[6] Enshirah D. Adsorption of heavy metals on functionalized-mesoporous silica. Microporous Mesoporous Mater. 2017; 247: 145-157. https://doi.org/10.1016/j.micromeso.2017.03.050
[7] Rasheed T, Ahmad N., Nabeel F, Anwar MT, Bilal M. Metal-organic frameworks for removal of heavy metals. In Nano-Bioremediation: Fundamentals and Applications (pp. 455-476). Elsevier; 2021 https://doi.org/10.1016/B978-0-12-823962-9.00014-3
[8] Guo L, Biao Z, Jing L, Zeying W, Shixing W, Tu H, Libo Z. A systematic review of metal organic frameworks materials for heavy metal removal: Synthesis, applications and mechanism. Chem Eng J. 2023; 460: 141710. https://doi.org/10.1016/j.cej.2023.141710
[9] Eman AA, Reyad A. Al Dwairi ZS, Removal of nickel (II) ions from water by Jordan natural zeolite as sorbent material. J Saudi Chem Soc. 2021; 25: https://doi.org/10.1016/j.jscs.2021.101233
[10] Gopal R, Neelam C, Tapan A, Nickel as a Pollutant and its Management. Int Res J Environment Sci. 2014; 3(10): 94-98. https://www.isca.me/IJENS/Archive/v3/i10/15.ISCA-IRJEvS-2014-189.pdf
[11] Senthil KP, Ramakrishnan K, Gayathri R, Removal of nickel (II) from aqueous solutions by ceralite IR 120 cationic exchange resins. J Eng Sci Technol. 2010; 5(2): 232-243. https://jestec.taylors.edu.my/Vol%205%20Issue%202%20June%2010/Vol_5(2)_232_243_P%20SENTHIL%20KUMAR.pdf
[12] Оleg LD, Irina RV, Galina ZS, Konstantin MN, Inna BV, Yulia KV, Alexander GY, Boris GP, Zilara FA, Coagulation removal of nickel (II) ions by ferric chloride. Water Environ Res. 2022; 94: e10827. https://doi.org/10.1002/wer.10827
[13] Jaroslav Černi Water Institute, Conceptual Design for WTP Zlatibor, 2016. (Technical documentation in Serbian)
[14] Jaroslav Černi Water Institute, Design for Constriction Permit for WTP Zlatibor, 2016. (Technical documentation in Serbian)
[15] Zane Satterfield PE, Jar Testing. Tech Brief, Publisher by The National Environmental Services Center. 2005; Volume 5 (1). https://www.nesc.wvu.edu/files/d/3cf372e5-ba40-450c-a3ad-cd774f4c3345/jar-testing.pdf
[16] Nadem ZM, Nadeem R, Asif HM, Biosorption of nickel from protonated rice bran. J Hazard Mater. 2007; 143: 478–85. https://doi.org/10.1016/j.jhazmat.2006.09.055
[17] Dash RR, Balomajumder C, Kumar A. Removal of cyanide from water and wastewater using granular activated carbon. Chem Eng J. 2009; 146: 40-413. https://doi.org/10.1016/j.cej.2008.06.021
[18] Inc. Metcalf & Eddy, Tchobanoglou G, Stensel H, Tsuchihashi R, Burton F, Abu-Orf M, Bowden G, Pfrang W. Wastewater Engineering: Treatment and Resource Recovery. 5th ed., New York, NY, USA: McGraw-Hill Education; 2014: 634. https://www.amazon.com/Wastewater-Engineering-Treatment-Resource-Recovery/dp/0073401188
[19] Jaroslav Černi Water Institute, Preliminary Design for WTP Zlatibor, 2016. (Technical documentation, in Serbian)