Efficiency evaluation of a natural material for removal of cationic oxazine and anionic azo dyes from aqueous solutions Original scientific paper
Main Article Content
Abstract
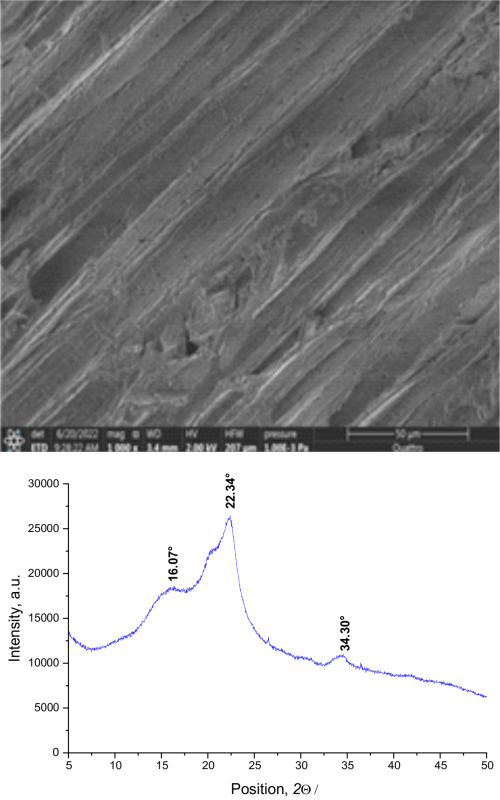
The current work involves studying the adsorption process of brilliant cresyl blue (BCB) and methyl orange (MeO) dyes using local pumpkin seed husks (LPSH). The LPSH adsorbent was analysed by using Fourier transform infrared spectroscopy, scanning electron microscopy with energy dispersive x-ray spectroscopy, X-ray diffraction and Brunauer-Emmett-Teller analyses. The descriptive analysis of the morphology of LPSH revealed a heterogeneous surface, while the structural analysis showed the presence of functional groups typical of lignocellulosic structures and it was confirmed that the mesoporous surface of the adsorbent had a specific surface area of ~1.53 m2 g-1. The adsorption isotherm studies suggested that the Langmuir model best described the adsorption of MeO, while the Freundlich model is more suitable for describing the adsorption of BCB. According to the thermodynamic analyses, the adsorption of BCB was exothermic and spontaneous, whereas the adsorption of MeO was endothermic and non-spontaneous. The results of evaluating the efficiency of the LPSH adsorbent showed that the maximum adsorption capacities are ~81 mg g-1 for the BCB dye and ~8.2 mg g-1 for the MeO dye.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
References
[1] Chaudhry FN, Malik MF. Factors Affecting Water Pollution. A Rev J Ecosyst Ecography. 2017; 7(225): 1-3. https://doi.org/10.4172/2157-7625.1000225
[2] Hussain S, Khan N, Gul S, Khan S, Khan H. Contamination of Water Resources by Food Dyes and Its Removal Technologies. In Water Chemistry. Eds; Intech Open London, UK, 2020. https://doi.org/10.5772/intechopen.90331
[3] Iwuozor KO, Ighalo JO, Emenike EC, Ogunfowora LA, Igwegbe CA. Adsorption of methyl orange: A review on adsorbent performance. Curr Res Green Sustain Chem. 2021; 4: 100179. https://doi.org/10.1016/j.crgsc.2021.100179
[4] Al-Gubury HY, Alteemi HS, Saad AM , Al-Shamary RR. Removal of Hazardous Brilliant Cresyl Blue Dye Utilizing Aluminum Oxide as Photocatalyst. Indones J Chem. 2019; 19(2): 292-297. https://doi.org/10.22146/ijc.30135
[5] Alhujaily A., Yu H., Zhang X. Ma F. Adsorptive removal of anionic dyes from aqueous solutions using spent mushroom waste. Appl Water Sci. 2020; 10: 183. https://doi.org/10.1007/s13201-020-01268-2
[6] Cako E, Gunasekaran KD, Soltani RDC, Boczkaj G. Ultrafast degradation of brilliant cresyl blue under hydrodynamic cavitation based advanced oxidation processes (AOPs). Water Resour Ind. 2020; 24: 100179. https://doi.org/10.1016/j.wri.2020.100134
[7] Adegoke KA, Bello OS. Dye sequestration using agricultural wastes as adsorbents. Water Resour Ind. 2015; 12: 8-24. https://doi.org/10.1016/j.wri.2015.09.002
[8] Zulti F, Setiadewi N, Waluyo A, & Susanti E. Removal pollutants in textile wastewater using unmodified rice husk. E3S Web Conf. 2024; 483: 02008. https://doi.org/10.1051/e3sconf/202448302008
[9] Yetgin S, Amlani M. Agricultural low-cost waste adsorption of methylene blue and modelling linear isotherm method versus nonlinear prediction. Clean Technol Environ Policy. 2024. https://doi.org/10.1007/s10098-024-02928-6
[10] Alivio RKO, Go AW, Angkawijaya AE, Santoso SP, Gunarto C, Soetaredjo FE. Extractives-free sugarcane bagasse as adsorbent for the removal of Rhodamine B (Basic Violet 10) with high capacity and reusability. J Ind Eng Chem. 2023; 124: 175-200. https://doi.org/10.1016/j.jiec.2023.04.007
[11] Ad C, Benalia M, Laidani Y, Elmsellem H, Henini G, Nouacer I, Djedid M. Kinetics, thermodynamics and equilibrium evaluation of adsorptive removal of iron from aqueous solution onto Algerian biosorbent “LUFFA CYLINDRICA.” J Mater Environ Sci. 2016; 7(1): 319-30. https://www.jmaterenvironsci.com/Document/vol7/vol7_N1/34-JMES-1968-2012-Ad.pdf
[12] Frey DD, Wang H. Adaptive One-Factor-at-a-Time Experimentation and Expected Value of Improvement. Technometrics. 2006; 48(3): 418-431. https://doi.org/10.1198/004017006000000075
[13] Awoyale AA, Lokhat D. Experimental determination of the effects of pretreatment on selected Nigerian lignocellulosic biomass in bioethanol production. Sci Rep. 2020; 11: 557. https://doi.org/10.1038/s41598-020-78105-8
[14] Khalfaoui A, Khelifi MN, Khelfaoui A, Benalia A, Derbal K, Gisonni C, Crispino G, Panico A A. The Adsorptive Removal of Bengal Rose by Artichoke Leaves: Optimization by Full Factorials Design. Water. 2022; 14(14): 2251. https://doi.org/10.3390/w14142251
[15] Ishak WHW, Ahmad I, Ramli S, Amin MCIM. Gamma Irradiation-Assisted Synthesis of Cellulose Nanocrystal-Reinforced Gelatin Hydrogels. Nanomaterials. 2018; 8: 749. https://doi.org/10.3390/nano8100749
[16] Kali A, Amar A, Loulidi I, Hadey C, Jabri M, Alrashdi AA, Lgaz H, Sadoq M. Efficient Adsorption Removal of an Anionic Azo Dye by Lignocellulosic Waste Material and Sludge Recycling into Combustible Briquettes. Colloids Interfaces. 2022; 6: 22. https://doi.org/10.3390/colloids6020022
[17] Purbasari A, Ariyanti D, Sumardiono S, Masyaroh M, Salsabila TR. Physical properties and structural characteristics of alkali modified fly ash. J Phys Conf Ser. 2021; 1912: 012012. https://doi.org/10.1088/1742-6596/1912/1/012012
[18] Horvat G, Pantic M, Knez Z, Novak Z. A Brief Evaluation of Pore Structure Determination for Bioaerogels. Gels. 2022; 8(7): 438. https://doi.org/10.3390/gels8070438
[19] Wawrzkiewicz M, Hubicki Z. Removal of tartrazine from aqueous solutions by strongly basic polystyrene anion exchange resins. J Hazard Mater. 2009; 164: 502-509. https://doi.org/10.1016/j.jhazmat.2008.08.021
[20] Estefan E, Elystia S, Kuan WH, Sasmita A. Removal of methyl orange textile dye using magnetic chitosan microspheres adsorbent. Water Pract Technol. 2023; 18(12): 3280-3290. https://doi.org/10.2166/wpt.2023.201
[21] Pourreza N, Mirzajani R, Behbahani MT. Removal of brilliant cresyl blue from aqueous solutions using modified zirconia nanoparticles as an adsorbent under ultrasonic action. Desalin Water Treat. 2016; 57: 28999-9006. https://doi.org/10.1080/19443994.2016.1193060
[22] Kurniawati D, Sari TK, Adella F, Sy S. Effect of Contact Time Adsorption of Rhodamine B, Methyl Orange and Methylene Blue Colours on Langsat Shell with Batch Methods. 1st Int. Conf. Chem. Sci. Educ. (ICChSE), J Phys Conf Ser. 2021; 1788: 012008. https://doi.org/10.1088/1742-6596/1788/1/012008
[23] Wong S, AbdGhafar N, Ngadi N, Razmi FA, Inuwa IM, Mat R, Amin NS. Effective removal of anionic textile dyes using adsorbent synthesized from cofee waste. Sci Rep. 2020; 10: 2928. https://doi.org/10.1038/s41598-020-60021-6
[24] Freundlich H, Helle WJ. The Adsorption of cis- and trans-Azobenzene. J Am Chem Soc. 1939; 61(8): 2228-30. https://doi.org/10.1021/ja01877a071
[25] Langmuir I. The adsorption of gases on plane surfaces of glass. J Am Chem Soc. 1918; 40: 1361-402. https://doi.org/10.1021/ja02242a004
[26] Badri N, Zbair M, Sahibed-Dine A, Chhiti Y, Khamliche L, Bensitel M. Adsorption of Cationic Dyes by Waste Biomass Treated by Phosphoric Acid. J Mater Environ Sci. 2018; 9: 1636-1644. https://doi.org/10.26872/jmes.2018.9.6.182
[27] Janet A, Kumaresan R, Uma Maheswari S. Adsorption of Anionic and Cationic Dyes onto Granular Activated Carbon. Middle-East J Sci Res. 2015; 23(2): 308-17. https://doi.org/10.5829/idosi.mejsr.2015.23.02.22114
[28] Saha P, Chowdhury S. Insight into adsorption thermodynamics. Thermodynamics. 2011; 16: 349-64. https://doi.org/10.5772/13474
[29] Adheem HM, Jasim LS. Preparation and Characterization of a three-component hydrogel composite and study of kinetic and thermodynamic applications of adsorption of some positive and negative dyes from their aqueous solutions. 2nd Int. Sci. Conf. Al-Ayen Univ. IOP Conference Series: IOP Conf Ser Mater Sci Eng. 2020; 928: 052027. https://doi.org/10.1088/1757-899X/928/5/052027
[30] Mandal B, Ray SK. Removal of safranine T and brilliant cresyl blue dyes from water by carboxy methyl cellulose incorporated acrylic hydrogels: Isotherms, kinetics and thermodynamic study. J Taiwan Inst Chem Eng. 2016, 60: 313-327. https://doi.org/10.1016/j.jtice.2015.10.021
[31] Amara-Rekkab A. Efficient Removal of Brilliant Cresyl Blue from Solution Using Inula racemosa: Experimental Insights, DFT Modeling, and Docking Simulation. Sep. Sci Technol. 2024; 9(41): e202403331. https://doi.org/10.1002/slct.202403331
[32] Gong R, Li M, Yang C, Sun Y, Chen J. Removal of cationic dyes from aqueous solution by adsorption on peanut hull. J Hazard. Mater. 2005; 121(1-3):247-250. https://doi.org/10.1016/j.jhazmat.2005.01.029
[33] Hambisa AA, Regasa MB, Ejigu HG, & Senbeto CB. Adsorption studies of methyl orange dye removal from aqueous solution using Anchote peel-based agricultural waste adsorbent. Appl Water Sci. 2023; 13(24). https://doi.org/10.1007/s13201-022-01832-y
[34] Dakhil IH. Recycling of Agriculture Wastes for Efficient Removal of Methyl Orange Dye Using Batch Adsorption Unit. IOP Conf. Ser.: Mater Sci Eng. 2020; 881(1): 012186. https://doi.org/10.1088/1757-899X/881/1/012186