Synthesis, characterization and electrochemical properties of cobalt-doped phosphate tungsten heteropoly acid and its bronze Original scientific paper
Main Article Content
Abstract
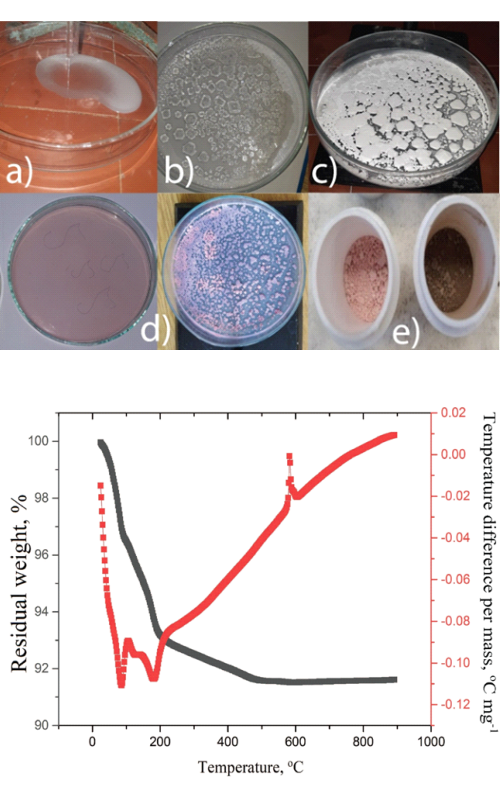
Heteropoly acids and their compounds are a fascinating class of multifunctional materials for use in various fields: medicine, magnetism, catalysis and nonlinear optics, as well as for electrochemistry and battery materials. This study used tungsten-phosphate heteropoly acid to synthesize and characterize its Co doped salt (Co-PWA) and tungsten-phosphate bronze (Co-PWB). Thermal analysis was used to determine Co-PWA salt phase transition into
Co-PWB bronze occurring at 588 °C. Both samples were further characterized using Fourier transform infrared spectroscopy, X-ray powder diffraction and scanning electron microscopy containing energy dispersive X-ray spectroscopy, and by use of electrochemical examinations. Cyclic voltammetry (as a rapid analytical method) showed that both materials yielded low capacities in an aqueous solution of LiNO3. However, a “slow” analytical method, chronopotentiometry, in which more places of a crystal lattice are occupied with ions (as compared to cyclic voltammetry), yielded solid and stable discharge capacity, making Co-PWB attractive as a potential electrode material for aqueous Li-ion batteries. The results obtained fill the gap in the scientific literature dealing with similar materials.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-66/2024-03/200026;451-03-66/2024-03/200051;451-03-66/2024-03/200146;451-03-65/2024-03/200126 -
HORIZON EUROPE European Innovation Council
Grant numbers 101115149 -
Office of Naval Research Global
Grant numbers N62902-22-1-2024
References
[1] Kamiya Y, Okuhara T, Misono M, Miyaji A, Tsuji K, Nakajo T. Catalytic chemistry of supported heteropolyacids and their applications as solid acids to industrial processes. Catal Surv Asia. 2008; 12: 101-113. https://doi.org/10.1007/s10563-008-9043-7
[2] Chikin AI, Chernyak AV, Jin Z, Naumova YS, Ukshe AE, Smirnova NV, Volkov VI, Dobrovolsky YA. Mobility of protons in 12-phosphotungstic acid and its acid and neutral salts. J Solid State Electrochem. 2012; 16: 2767-2775. https://doi.org/10.1007/s10008-012-1687-6
[3] Kourasi AM, Wills RGA, Shah AA, Walsh FC. Heteropolyacids for fouel cell applications. Electrochim Acta. 2014; 127: 454-466. https://doi.org/10.1016/j.electacta.2014.02.006
[4] Imofeeva MN, Maksimov GM, Likholobov VA. Acidity of solutions of heteropoly acids with various structures and compositions. Kinetic Catal. 2001; 42: 30-34. https://doi.org/10.1023/A:1004892411282
[5] Kima JD, Hayashi S, Mori T, Honma I. Fast proton conductor under anhydrous condition synthesized from 12-phosphotungstic acid and ionic liquid. Electrochim Acta. 2007; 53: 963-967. https://doi.org/10.1016/j.electacta.2007.08.009
[6] Schneea J, Devredb F, Gaigneauxb EM, Vimonta A, Assessing the dispersion of supported H3PW12O40 catalysts: No longer a hurdle thanks to in situ IR upon pyridine adsorption. Appl Catal. 2019; 578: 116-121. https://doi.org/10.1016/j.apcata.2019.03.022
[7] Brown GM, Noe-Spirlet MR, Busing WR, Levy HA. Dodecatungstophosphoric acid hexahydrate, (H5O2+)3(PW12O403-). The true structure og Keggins pentahydrate from single-crystal X-ray and neutron diffraction data. Acta Cryst. 1977; B33: 1038-1046. https://doi.org/10.1107/S0567740877005330
[8] Spirlet MR, Busing WR. Dodecatungstophosphoric acid-21-water by neutron diffraction. Acta Cryst. 1978; 34(3): 907-910. https://doi.org/10.1107/S0567740878004306
[9] Kremenović A, Spasojević-de Biré A, Dimitrijević R, Sciau P., Mioč UB, Colomban Ph. Keggin’s ion structural modification and expansion of dodecatungstophosphoric acid hexahydrate induced by temperature treatment: In situ X-ray powder diffraction and Raman investigations. Solid State Ion. 2000; 132: 39-53. https://doi.org/10.1016/S0167-2738(00)00727-X
[10] Davidović M, Čajkovski T, Colomban Ph, Mioč UB, Likar-Smiljanić V, Čajkovski D, Biljić R, Nedić Z. The influence of monovalent and bivalent cations on the electrical properties of 12-tungstophosphoric acid salts. Solid State Ion. 2005; 176: 2881-2885. https://doi.org/10.1016/j.ssi.2005.09.020
[11] Kulesza PJ, Rutkowska IA, Janiszewska C, Noto VD, Vezzu K, Negro E. Development and characterization of polyoxometallate-based systems for aqueous redox flow batteries. Meet Abstr. 2022; MA2022-01: 1999. https://doi.org/10.1149/MA2022-01481999mtgabs
[12] Ammam M, Fransaer J. Ionic liquid-heteropolyacid: Synthesis, characterization, and supercapacitor study of films deposited by electrophoresis. J Electrochem Soc. 2011; 158: A14. https://doi.org/10.1149/1.3507254
[13] Mioč UB, Dimitrijević RŽ, Davidović M, Nedić ZP, Mitrović MM, Colomban Ph. Thermally inducted phase transformations of 12-tungstophosphoric acid 29-hydrate:synthesis and characterization of PW8O26-type bronzes. J Mater Sci. 1994; 29: 3705-3718. https://doi.org/10.1007/BF00357338
[14] Dimitrijević RŽ, Colomban Ph, Mioč UB, Nedić Z, Todorović MR, Tjapkin N, Davidović M. Synthesis, conductivity and structural characterization of phosphorous bronzes originating from heteropolyacids. Relation with similar proton containing phases. Solid State Ion. 1995; 77: 250-256. https://doi.org/10.1016/0167-2738(94)00310-O
[15] Maksimović TV, Maksimović JP, Joksović LjG, Nedić ZP, Pagnacco MC. Oscilatorna reakcija kao sistem detektor za dopirane i nedopirane fosfat-volframove bronze. Hem Ind. 2018; 72(5): 275-283. (in Serbian) https://doi.org/10.2298/HEMIND180402018M
[16] Sweedler AR, Raub ChJ, Matthias BT. Superconductivity of the alkali tungsten bronzes. Phys Lett. 1965; 15(2): 108-109. https://doi.org/10.1016/0031-9163(65)91292-8
[17] Brusetti R, Bordet P, Bossy J, Schober H, Eibl S. Superconductivity in the tungsten bronze RbxWO3 (0.20⩽ x⩽ 0.33) in connection with its structure, electronic density of states, and phonon density of states. Phys Rev. 2007; B76: 174511. https://doi.org/10.1103/PhysRevB.76.174511
[18] Yoon S, Jo C, Noh SY, Lee CW, Song H, Lee J. Development of a high-performance anode for lithium ion batteries using novel ordered mesoporous tungsten oxide materials with high electrical conductivity. PCCP. 2011; 13: 11060-11066. https://doi.org/10.1039/C1CP20940J
[19] Wasserman K, Pope MT, Salmen M, Dann JN, Lunk HJ. Thermal degradation of polyoxotungstates-an effective method for the preparation of tungsten bronzes. J Solid State Chem. 2000; 149: 378-383. https://doi.org/10.1006/jssc.1999.8556
[20] Dong X, Lu Y, Wu Z, Liu X, Tong Y. Photochromic hierarchical (NH4)xWO3 nanocrystals with bronze tunnel structure for energy-saving windows. Chem Eng J. 2023; 477: 147064. https://doi.org/10.1016/j.cej.2023.147064
[21] Acković J, Micić R, Nedić Z, Petrović T, Senćanski J, Pagnacco M, Tančić P. Synthesis, characterization and electrochemical properties of iron doped phosphate tungsten heteropoly acid (Fe-PWA) and its bronze (Fe-PWB): Comparative study. Sci Sinter. 2024; 56: 367-380. https://doi.org/10.2298/SOS230812053A
[22] Pagnacco M, Marković S, Potočnik J, Krstić V, Tančić P, Mojović M, Mojović Z. The influence of electrode constituents on hydrogen evolution reaction on phosphate W-and Mo bronze based electrodes. J Electrochem Soc. 2022; 169(10): 106508. https://doi.org/10.1149/1945-7111/ac96ab
[23] Maksimović JP, Maksimović TV, Nedić ZP, Pagnacco MC. The minor influence of calcium doped phosphate tungsten bronze on the Briggs-Rauscher reaction dynamics. Contemp Mater. 2018; 184-189. https://doi.org/10.7251/COMEN1802184M
[24] Maksimović TV, Maksimović JP, Tančić PI, Potkonjak NI, Nedić ZP, Joksović LjG, Pagnacco MC. A possible connection between phosphate tungsten bronzes properties and Briggs-Rauscher oscillatory reaction response. Sci Sinter. 2021; 53: 223. https://doi.org/10.2298/SOS2102223M
[25] Maksimović T, Tančić P, Maksimović J, Mara D, Ilić M, Van Deun R, Joksović Lj, Pagnacco M. Novel cerium and praseodymium doped phosphate tungsten bronzes: Synthesis, characterization, the behavior in the Briggs-Rauscher reaction and photoluminescence properties. Opt Mater. 2023; 143: 114125. https://doi.org/10.1016/j.optmat.2023.114125
[26] Vujković M, Nedić Z, Tančić P, Aleksić OS, Nikolić MV, Mioč U, Mentus S. Electrochemical lithiation/delithiation kinetics and capacity of phosphate tungsten bronze and its chemically pre-lithiated derivatives aqueous solutions. J Mater Sci. 2016; 51: 2481-2489. https://doi.org/10.1007/s10853-015-9560-5
[27] Rodriguez-Carvajal, J. Program Fullprof (Computer software). In Proceedings of the Abstract of 15th Conference of International Union of Crystallography, Satellite Meeting on Powder Diffraction, Toulouse, France, July 16-19th 1990; p. 127.
[28] Tančić P, Dimitrijević R, Poznanović M, Pačevski A, Sudar S. Crystal structure and chemical composition of ludwigite from Vranovac ore deposit (Boranja Mountain, Serbia). Acta Geol Sin-Engl. 2012; 86(6): 1524-1538. https://doi.org/10.1111/1755-6724.12020
[29] Tančić P, Kremenović A, Vulić P. Structural dissymmetrization of optically anisotropic Grs64±1Adr36±1Sps2 grandite from Meka Presedla (Kopaonik Mt., Serbia). Powder Diffr. 2020; 35(1): 7-16. https://doi.org/10.1017/S0885715619000897
[30] Tančić P, Kremenović A. Rietveld crystal structure refinement of a natural rhombohedral grossular-andradite garnet from Serbia. Geol Q. 2022; 66(7): 1639. http://dx.doi.org/10.7306/gq.1639
[31] Mioč U, Davidović M, Tjapkin N, Colomban Ph, Novak A. Equilibrium of the protonic species in hydrates of some heteropolyacids at elevated temperatures. Solid State Ion. 1991; 46: 103-109. https://doi.org/10.1016/0167-2738(91)90136-Y
[32] Ratajczak H, Barnes AJ, Bielański A, Lutz HD, Müller A, Pope MT. Vibrational Spectroscopy of Heteropoly Acids. In: Pope, MT, Müller A. (eds) Polyoxometalate Chemistry From Topology via Self-Assembly to Applications. Springer Dordrecht. 2001; 101-116. https://doi.org/10.1007/0-306-47625-8_8
[33] Jose da Silva M, Macedo de Oliveira C. Catalysis by Keggin heteropolyacid salts. Curr Catal. 2018; 7: 26-34. https://doi.org/10.2174/2211544707666171219161414
[34] Rocchiccioli-Deltcheff C, Fournier M, Franck R, Thouvenot R, Vibrational investigations of polyoxometalates. 2. Evidence for anion-anion interactions in molybdenum(VI) and tungsten(VI) compounds related to the keggin structure. Inorg Chem. 1983; 22: 207-216. https://doi.org/10.1021/ic00144a006
[35] Martinez-de la Cruz A, Rodriguez FEL, Rodriguez JI. Electrochemical lithium insertion in the phosphate tungsten bronze P8W12O52. Solid State Ion. 2005; 176: 2625-2630. https://doi.org/10.1016/j.ssi.2005.08.009
[36] Martínez-de la Cruz A, Rodríguez FEL. Electrochemical lithium insertion in (PO2)4(WO3)2m (2≤m≤10): Relation among the electrochemical insertion process and structural features. Electrochim Acta. 2009; 54: 3176-3183. https://doi.org/10.1016/j.electacta.2008.11.056
[37] Wang E, Greenblatt M. Lithium and sodium insertion reactions of phosphate tungsten bronzes. J Solid State Chem. 1987; 68: 38-44. https://doi.org/10.1016/0022-4596(87)90282-9