Natural deep eutectic solvents for turbidity removal from synthetic pharmaceutical wastewater Original scientific paper
Main Article Content
Abstract
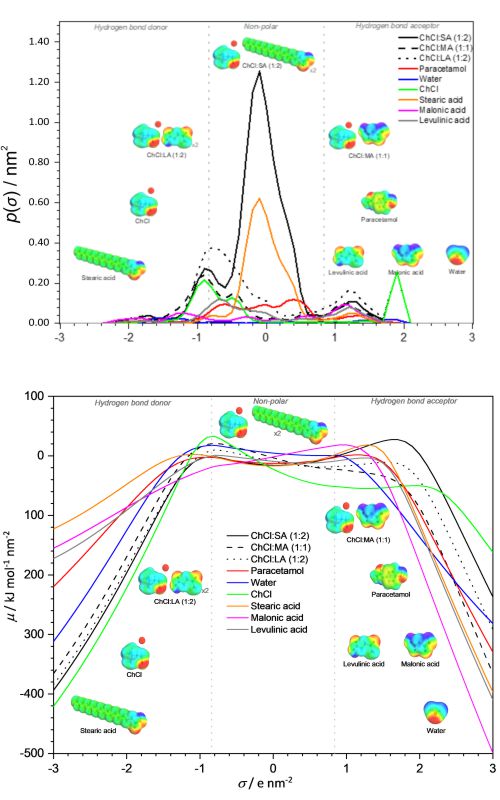
Water resource contamination by active pharmaceutical ingredient (API) wastes are among major environmental concerns. To prevent major disruptions of aquatic life, an efficient and environmental-friendly turbidity removal procedure of common contaminants such as paracetamol should be established. In this study, several natural deep eutectic solvents (NADESs) were screened to reduce the turbidity of simulated water contaminated with paracetamol to below the standard turbidity limit recommended by the National Water Quality Standards for Malaysia (SIRIM) (50 NTU). The optimum operating parameters (NADES dosage, stirring time and operating pH) were determined. Under optimized conditions, stearic acid-based NADES achieved the highest turbidity removal at 97.46%. The high coagulation performances were investigated based on molecular interaction using COSMO-RS σ-profile and σ-potential, and showed high affinity between the NADES compounds and paracetamol. Thus, NADESs are promising candidates for turbidity removal of paracetamol from water and are viable in further investigations for effluent treatment applications.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree to the following terms:
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Authors grant to the Publisher the following rights to the manuscript, including any supplemental material, and any parts, extracts or elements thereof:
- the right to reproduce and distribute the Manuscript in printed form, including print-on-demand;
- the right to produce prepublications, reprints, and special editions of the Manuscript;
- the right to translate the Manuscript into other languages;
- the right to reproduce the Manuscript using photomechanical or similar means including, but not limited to photocopy, and the right to distribute these reproductions;
- the right to reproduce and distribute the Manuscript electronically or optically on any and all data carriers or storage media – especially in machine readable/digitalized form on data carriers such as hard drive, CD-Rom, DVD, Blu-ray Disc (BD), Mini-Disk, data tape – and the right to reproduce and distribute the Article via these data carriers;
- the right to store the Manuscript in databases, including online databases, and the right of transmission of the Manuscript in all technical systems and modes;
- the right to make the Manuscript available to the public or to closed user groups on individual demand, for use on monitors or other readers (including e-books), and in printable form for the user, either via the internet, other online services, or via internal or external networks.
How to Cite
Funding data
-
Universiti Malaya
Grant numbers GPF015a-2023 -
Ministry of Higher Education, Malaysia
Grant numbers FRGS/1/2023/TK05/UKM/02/5
References
Crini G, Lichtfouse E. Advantages and disadvantages of techniques used for wastewater treatment. Environ Chem Lett. 2019; 17(1): 145-155. https://doi.org/10.1007/s10311-018-0785-9
Yao T, Gan Y, Li Q, Tan M, Shi X. Removal and recovery of triphenylmethane dyes from wastewater with temperature-sensitive magnetic ionic liquid aqueous two-phase system. J Clean Prod. 2021; 328: 129648. https://doi.org/10.1016/j.jclepro.2021.129648
Imdad S, Dohare RK. A Critical Review on Heavy Metals Removal Using Ionic Liquid Membranes from The Industrial Wastewater. Chem Eng Process. 2022; 173: 108812. https://doi.org/10.1016/j.cep.2022.108812
Sundararaman S, Aravind Kumar J, Deivasigamani P, Devarajan Y. Emerging pharma residue contaminants: Occurrence, monitoring, risk and fate assessment – A challenge to water resource management. Sci Total Environ. 2022; 825: 153897. https://doi.org/10.1016/j.scitotenv.2022.153897
Sgroi M, Anumol T, Vagliasindi FGA, Snyder SA, Roccaro P. Comparison of the new Cl2/O3/UV process with different ozone- and UV-based AOPs for wastewater treatment at pilot scale: Removal of pharmaceuticals and changes in fluorescing organic matter. Sci Total Environ. 2021; 765: 142720. https://doi.org/10.1016/j.scitotenv.2020.142720
Gadipelly C, Pérez-González A, Yadav GD, Ortiz I, Ibáñez R, Rathod VK, Marathe KV. Pharmaceutical Industry Wastewater: Review of the Technologies for Water Treatment and Reuse. Ind Eng Chem Res. 2014; 53(29): 11571-11592. https://doi.org/10.1021/ie501210j
Florindo C, Monteiro NV, Ribeiro BD, Branco LC, Marrucho IM. Hydrophobic deep eutectic solvents for purification of water contaminated with Bisphenol-A. J Mol Liq. 2020; 297: 111841. https://doi.org/10.1016/j.molliq.2019.111841
Florindo C, Lima F, Branco LC, Marrucho IM. Hydrophobic Deep Eutectic Solvents: A Circular Approach to Purify Water Contaminated with Ciprofloxacin. ACS Sustain Chem Eng. 2019; 7(17): 14739-14746. https://doi.org/10.1021/acssuschemeng.9b02658
Qu Q, Lv Y, Liu L, Row KH, Zhu T. Synthesis and characterization of deep eutectic solvents (five hydrophilic and three hydrophobic), and hydrophobic application for microextraction of environmental water samples. Anal Bioanal Chem. 2019; 411(28): 7489-7498. https://doi.org/10.1007/s00216-019-02143-z
Florindo C, Branco LC, Marrucho IM. Development of hydrophobic deep eutectic solvents for extraction of pesticides from aqueous environments. Fluid Phase Equilibr. 2017; 448: 135-142. https://doi.org/10.1016/j.fluid.2017.04.002
Ge D, Wang Y, Jianga Q, Dai E. A Deep Eutectic Solvent as an Extraction Solvent to Separate and Preconcentrate Parabens in Water Samples Using in situ Liquid-Liquid Microextraction. J Brazil Chem Soc. 2019; 30(6): 1203-1210. https://doi.org/10.21577/0103-5053.20190014
Elgharbawy AAM, Hayyan A, Hayyan M, Mirghani MES, Salleh HM, Rashid SN, Ngoh GC, Liew SQ, Nor MR, bin Mohd Yusoff MYZ, Alias Y. Natural Deep Eutectic Solvent-Assisted Pectin Extraction from Pomelo Peel Using Sonoreactor: Experimental Optimization Approach. Processes. 2019; 7(7). https://doi.org/10.3390/pr7070416
Al-Risheq DIM, Nasser MS, Qiblawey H, Ba-Abbad MM, Benamor A, Hussein IA. Destabilization of stable bentonite colloidal suspension using choline chloride based deep eutectic solvent: Optimization study. J Water Process Eng. 2021; 40: 101885. https://doi.org/10.1016/j.jwpe.2020.101885
Al-Risheq DIM, Nasser MS, Qiblawey H, Hussein IA, Benamor A. Choline chloride based natural deep eutectic solvent for destabilization and separation of stable colloidal dispersions. Sep Purif Technol. 2021; 255: 117737. https://doi.org/10.1016/j.seppur.2020.117737
Klamt A. COSMO-RS: From Quantum Chemistry to Fluid Phase Thermodynamics and Drug Design. 1st ed., Amsterdam, Netherlands: Elsevier; 2005.
Foong CY, Zulkifli MFM, Wirzal MDH, Bustam MA, Nor LHM, Saad MS, Abd Halim NS. COSMO-RS prediction and experimental investigation of amino acid ionic liquid-based deep eutectic solvents for copper removal. J Mol Liq. 2021; 333: 115884. https://doi.org/10.1016/j.molliq.2021.115884
Hu W, Shang Z, Wei N, Hou B, Gong J, Wang Y. Solubility of benorilate in twelve monosolvents: Determination, correlation and COSMO-RS analysis. J Chem Thermodyn. 2021; 152: 106272. https://doi.org/10.1016/j.jct.2020.106272
Khan AS, Ibrahim TH, Rashid Z, Khamis MI, Nancarrow P, Jabbar NA. COSMO-RS based screening of ionic liquids for extraction of phenolic compounds from aqueous media. J Mol Liq. 2021; 328: 115387. https://doi.org/10.1016/j.molliq.2021.115387